3M™ Skin and Nasal Antiseptic, 192401, 4 mL, 12 Each/Carton, 4Carton/Case
About the product
Designed to fit easily into your preoperative skin prep process, Skin and Nasal Antiseptic features a fast acting, pH balanced buffered formulation10 with a film forming polymer that increases persistence and helps reduce the risk of surgical site infections when part of a comprehensive preoperative protocol.
Product details
S. aureus is the leading cause of surgical site infections (SSIs), with 80% of infections from S. aureus coming directly from a patient s nasal flora. Fitting seamlessly into your preoperative process, Skin and Nasal Antiseptic is a simple, one time application that reduces skin flora by 99.9% within 10 minutes and maintains reduction for at least six hours.* As part of a comprehensive preoperative protocol, our skin and nasal antiseptic solution is an important tool to help reduce surgical site infections (SSIs) while supporting antibiotic stewardship.2
*When tested on the groin and abdomen. Reference: I2MS-05-010945
- Helps lowers the risk of surgical site infections for your patients with a one time application when used as part of a comprehensive preoperative protocol.1
- Skin and nasal antiseptic offers short and long term protection by reducing skin flora by 99.9% within ten minutes* while maintaining reduction for at least six hours.*
- Features a film forming, patented formula designed not to drip, does not contain alcohol and has demonstrated excellent acceptability.1,9
- The broad spectrum, fast acting antiseptic allows for wider implementation than antibiotic treatment and fits easily into your preoperative process.3
- Helps improve patient safety and protocol compliance without alcohol or antibiotics. Directly observed application of Skin and Nasal Antiseptic ensures compliance, unlike other methods addressing nasal decolonization.2
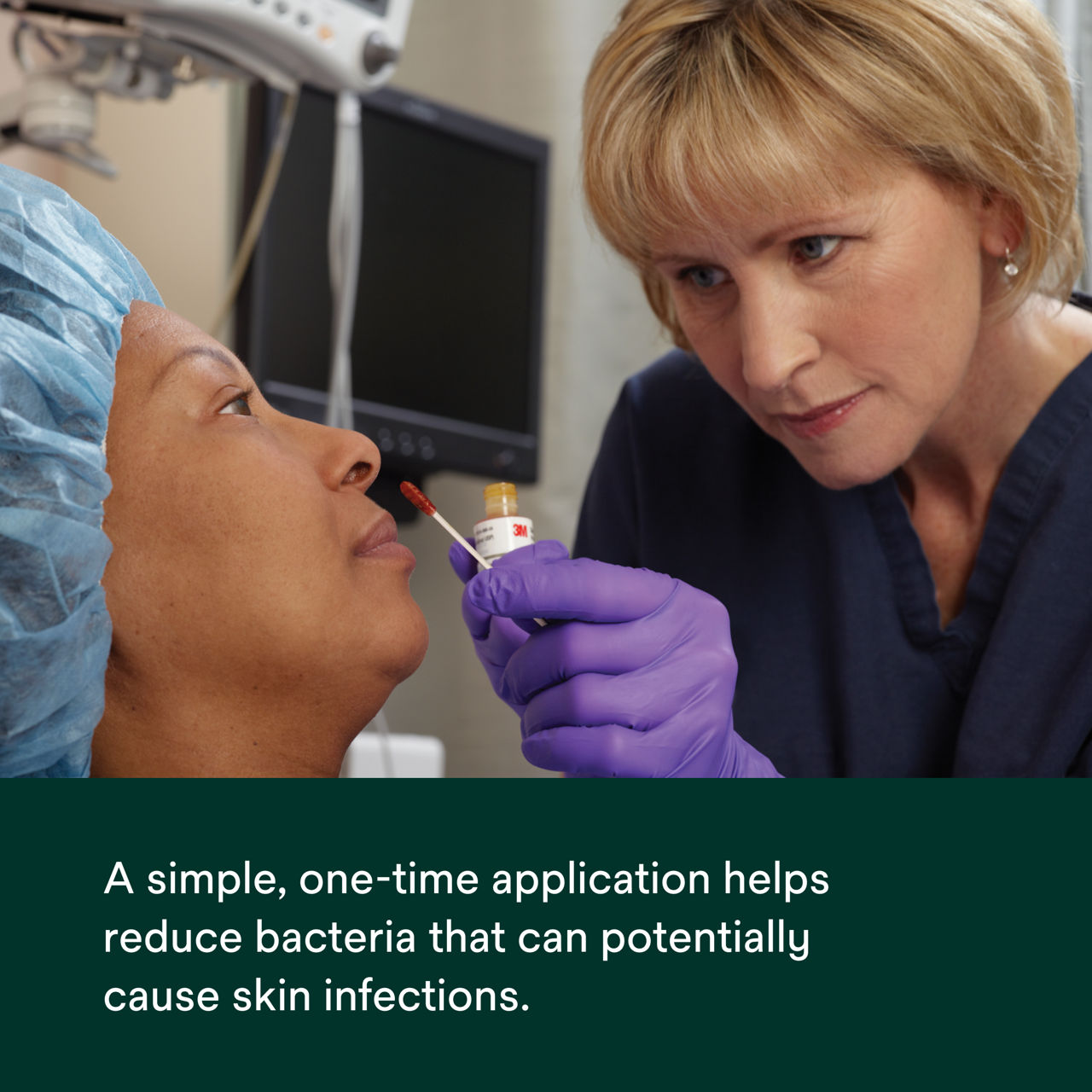
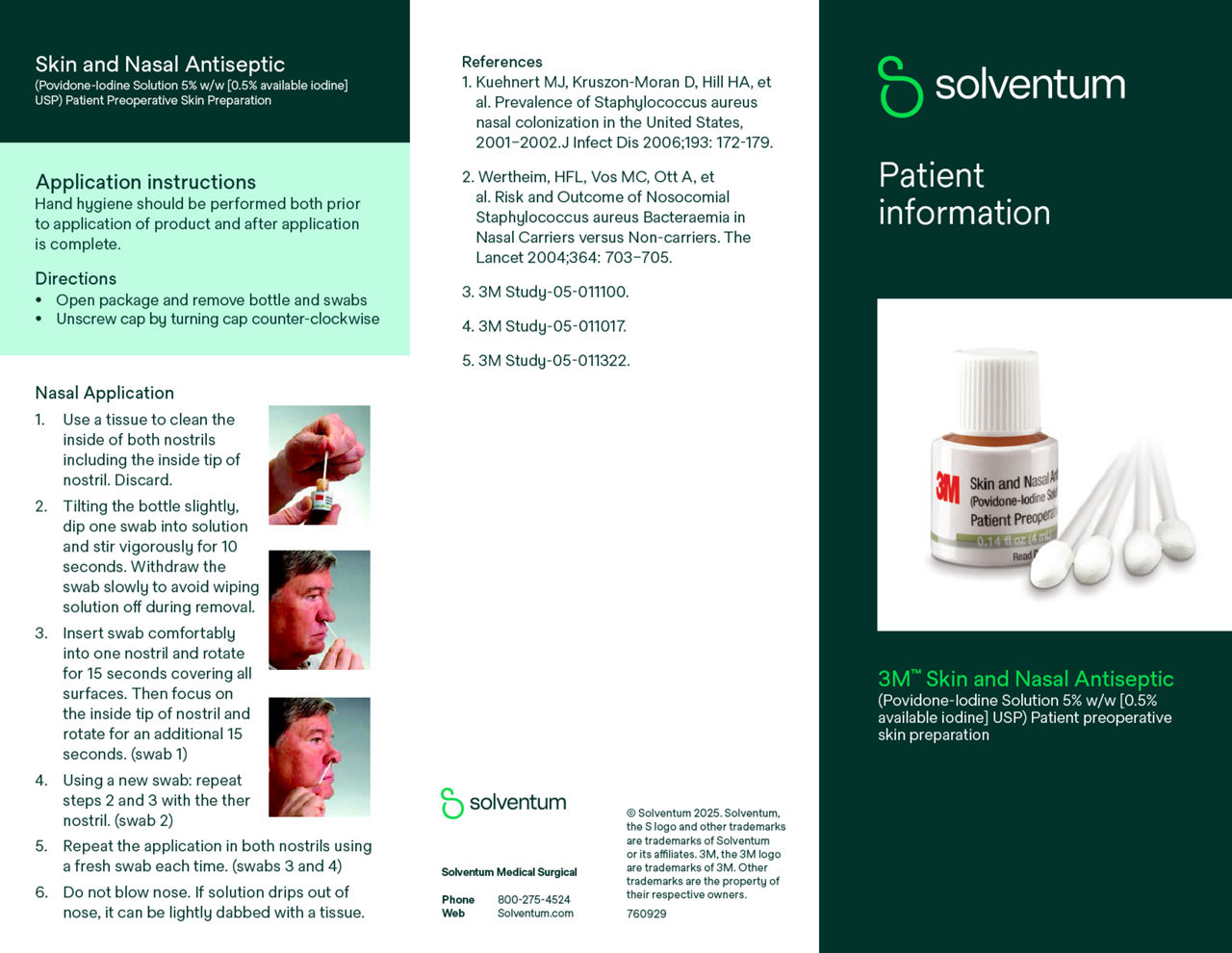
- Designed to be administered one hour before surgery to reduce bacteria in the nares.
*When tested on the groin and abdomen. Reference: I2MS 05-010945
Suggested Applications
- For preparation of skin prior to surgerya
- Helps reduce bacteria that can potential cause skin infections
Legal
1 Solventum Study 05-011100
2 Phillips M, Rosenberg A, Shopsin B, et al. Preventing surgical site infections: A randomized, open label trial of nasal mupirocin ointment and nasal povidone iodine solution. Infect Control Hosp Epidemiol 2014;35(7):826-832.
3 Bebko SP, Green DM, Awad SS. Effect of a preoperative decontamination protocol on surgical site infections in patients undergoing elective orthopedic surgery with hardware implantation. JAMASurg. Published online March 04, 2015. doi:10.1001/jamasurg.2014.3480.
4 Flynn N, Carr M. Skin and Nasal Antiseptic use in the prevention of post operative infections. Presented at the SHEA Conference, Orlando, FL, May 2015.
5 Brown L, Shelly M, Greene L, et al. The effect of universal intranasal povidone iodine antisepsis on total joint replacement surgical site infections. Presented at the APIC National Conference,Anaheim, CA, June 2014.
6 Waibel ML. Revisiting process improvement for total joint arthroplasty SSI. Presented at the APIC National Conference, Fort Lauderdale, FL, June 2013.
7 Hogenmiller JR, Hamilton J, Clayman T, et al. Preventing orthopedic total joint replacement SSIs through a comprehensive best practice bundle/checklist. Presented at the APIC National Conference, Baltimore, MD, June 2011.
8 Osborn N, Reynolds L. Embedding an Infection Preventionist (IP) in the OR. Presented at the AORN Surgical Conference and Expo, Denver, CO, March 2015.
9 Solventum Study 05-011017
10 Solventum data on file: EM 05-149605.
11 Torres EG, Lindmair Snell JM, Langan JW, Burnikel BG. Is preoperative nasal povidone iodine as efficient and cost effective as standard methicillin resistant Staphylococcus aureus screening protocol in total joint arthroplasty? J Arthroplasty. 2016; 31: 215–218.
Product specifications
| categoryName |
categoryName
Patient Skin Prep |