Solventum Prevena Therapy patient information
What is Prevena Therapy?
Protecting your incision is important to your recovery, which is why your doctor has prescribed you a Solventum™ Prevena™ Therapy System — a portable, disposable unit — for management of your surgical incision. Solventum™ Prevena™ 125 Therapy Unit and Solventum™ Prevena™ Plus 125 Therapy Unit manage the environment of closed surgical incisions and remove fluid away from the surgical incision via the application of -125mmHg continuous negative pressure. The Prevena Therapy units when used with Prevena dressings are the first devices indicated by the FDA to help reduce superficial surgical site infections in patients at high risk for postoperative infections in Class I and Class II wounds.*
*The effectiveness of Prevena Therapy in reducing the incidence of SSIs and seroma in all surgical procedures and populations has not been demonstrated. Please see the full indications for use and limitations below.
Start your Prevena Therapy journey
Patient Video
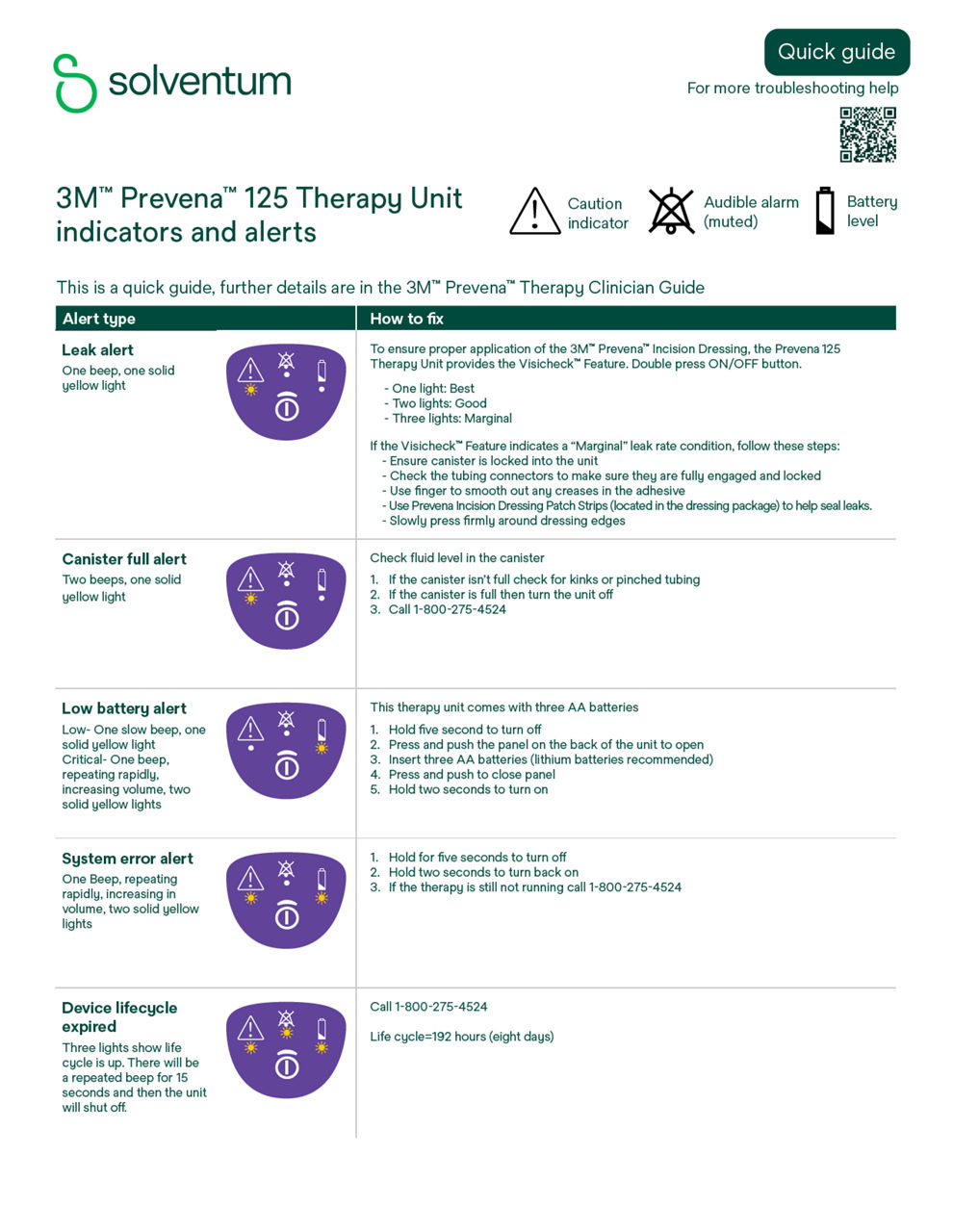
Discover more about your Prevena Therapy Unit - how it works, everyday tips for wearing and caring for your system, and how to read and troubleshoot the alerts on your unit.
Solventum Prevena 125 Therapy Unit
FAQs
Refer to the Prevena Therapy Patient Guide (PDF, 770.19 KB) or Alert Guide (PDF, 1.50 MB) or contact your health care provider if you have questions.
Wear the system for as long as your health care provider has ordered, at which time the dressing will be removed by your doctor. The therapy unit will turn off automatically at 7 days.
The dressing is not typically changed. Follow your health care provider's instructions.
This portable unit should always be kept turned on for the time your health care provider has instructed. The unit can be worn under or over your clothing and includes a carrying case with adjustable straps, so you can use it multiple ways.
Keep your therapy unit in a safe place where tubing will not become kinked or pinched, and it cannot be pulled off a table or dropped onto the floor.
This therapy unit comes with three AA size batteries and cannot be recharged. It is recommended that you keep extra batteries on hand. If the batteries run out, consult your Prevena Therapy Patient Guide on how to replace the batteries.
If cleared by your health care provider, a quick, light shower is okay. Keep the therapy unit away from direct water spray. Do not submerge dressing in water. When towel drying, be careful not to disrupt the dressing.
Patient Video
Discover more about your Prevena Therapy Unit - how it works, everyday tips for wearing and caring for your system, and how to read and troubleshoot the alerts on your unit.
Solventum Prevena 125 Therapy Unit
FAQs
Every patient is unique, and your health care provider should provide you with clear information about the length of your treatment. You should wear the system for as long as your health care provider has ordered. As a guide, the Prevena 125 Therapy Unit for use on closed surgical incisions will last up to 7 days. The Prevena Plus 125 Therapy Unit which can be used on both closed incisions and open wounds will last up to 14 days.
The frequency of dressing changes will be dependent on your treatment. Your health care provider should provide you with information about whether your dressings will require changing, and if so, how often. Please ensure you follow your health care provider's instructions. If you are undergoing treatment for an open wound with Prevena Plus and V.A.C. Dressings, dressing changes should be every 48-72 hours. If you are treating a closed surgical incision with Prevena Dressings, then the dressing is not typically changed, and will last up to 7 days. If you are treating a closed surgical incision with Prevena Restor Dressings, then the dressing is not typically changed, and will last up to 14 days.
Refer to the Prevena Therapy Patient Guide (PDF, 770.19 KB) or Alert Guide (PDF, 1.50 MB) Or if you have any questions, call our Customer Service team on freephone 0800 980 8880, or freephone 1800 33 33 77 if in Ireland.
This portable unit should always be kept turned on for the time your doctor has instructed. The unit can be worn under or over your clothing and includes a carrying case with adjustable straps, so you can use it multiple ways.
Keep your therapy unit in a safe place where tubing will not become kinked or pinched, and it cannot be pulled off a table or dropped onto the floor.
Prevena Plus 125 Therapy Unit has a rechargeable battery and comes with a charger. Bring your charger with if you are gone for extended periods of time. For convenience, consider charging your therapy unit battery while you are sleeping.
If cleared by your health care provider, a quick, light shower is okay. Keep the therapy unit away from direct water spray. Do not submerge dressing in water. When towel drying, be careful not to disrupt the dressing.
Prevena Therapy for a supported surgical recovery
If you're preparing for surgery, it's crucial to think about protecting your post-surgery incision to aid your recovery. Your incision will be closed with methods like stitches or staples, and yet, surgical incisions carry risks that may affect your healing:
Proper incision care is vital for a timely recovery and maintaining your quality of life. Prevena Therapy system can be a part of your care plan. This easy-to-use, disposable system applies gentle negative pressure to protect and help manage your incision and help reduce superficial surgical site infections in Class II and Class III wounds for high-risk patients.* Prevena Therapy works by:
- Incision management: Creates a barrier to external contaminants and protects the incision site
- Fluid management: Removes fluids and infectious materials
- Incision support: Helps keep incision edges together
Patients with delicate skin, acrylic and/or hydrocolloid adhesive or silver sensitivities/allergies, or those at increased risk of bleeding should use Prevena Therapy cautiously. For comprehensive details, please consult the patient guide, as well as your doctor.
*For patients at high-risk for post-operative complications in Class I and Class II wounds. The effectiveness of Prevena Therapy in reducing the incidence of SSIs and seroma in all surgical procedures and populations has not been demonstrated. Please see the full indications for use and limitations below.
NOTE:
Specific indications, limitations, contraindications, warnings, precautions and safety information exist for these products and therapies. Please consult a clinician and product instructions for use prior to application. Rx only.
Indication(s) For Use / Intended Use: US FDA Cleared: Only for Use in the United States:
Prevena Dressing used with Prevena Therapy Units: PREVENA™ 125 and PREVENA PLUS™ 125 Therapy Units manage the environment of closed surgical incisions and remove fluid away from the surgical incision via the application of -125mmHg continuous negative pressure. When used with legally marketed compatible PREVENA™ dressings for up to seven days, PREVENA™ 125 and PREVENA PLUS™ 125 Therapy Units are intended to aid in reducing the incidence of seroma; and in patients at high risk for post-operative infections, aid in reducing the incidence of superficial surgical site infection in Class I and Class II wounds.
Limitations:
- The device is not intended to treat surgical site infection or seroma.
- Safety and effectiveness in pediatric population (<22 years old) have not been evaluated.
- Safety and effectiveness in Class III (Contaminated) and Class IV (Dirty/Infected) wounds have not been demonstrated. Furthermore, Class IV surgical wounds are not expected to be closed primarily, and the subject device should only be used on closed surgical incisions.
- The device has not been demonstrated to reduce deep incisional and organ space surgical site infections.
- The device has not been demonstrated to be effective in reducing the incidence of surgical site infection and seroma in all surgical procedures and patient populations; therefore, the device may not be recommended for routine use to reduce the incidence of surgical site infection and seroma.
- Please refer to the ‘Summary of Clinical Information’ section for the specific surgical procedures and patient populations included in the clinical studies. Surgeons should continue to follow the ‘Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection’4 and the ‘American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines’5 for best practices in preventing surgical site infection. US FDA Cleared: Dressings/ Systems (Prevena Dressings used with compatible Solventum NPWT units - ActiVAC, Ulta, and RX4) and applicable OUS countries that leverage US Indication: The PREVENA™, PREVENA PLUS™, PREVENA DUO™, and PREVENA RESTOR™ Incision Management Systems are intended to manage the environment of surgical incisions that continue to drain following sutured or stapled closure by maintaining a closed environment and removing exudates via the application of negative pressure wound therapy.
References:
- Zimlichman E, Henderson D, Tamir, et al. Health care-associated Infections a meta-analysis of costs and financial impact on the U.S. health care system. JAMA Intern ed.2013;173(22):20-46.
- Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated Infections. N Engl JMed. 2014;370:1198-208.
- Shepard J, Ward W, Milstone A, et al. Financial impact of surgical site infections on hospitals. The hospital management perspective. JAMA Surg. 2013;148(10):907-914. doi:10.1001/jamasurg.2013.2246
- Berríos-Torres, S. I., Umscheid, C. A., Bratzler, D. W., Leas, B., Stone, E. C., Kelz, R. R., Reinke, C. E., Morgan, S., Solomkin, J. S., Mazuski, J. E., Dellinger, E. P., Itani, K. M., Berbari, E. F., Segreti, J., Parvizi, J., Blanchard, J., Allen, G., Kluytmans, J. A., Donlan, R., & Schecter, W. P. (2017). Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surgery, 152(8), 784. https://doi.org/10.1001/jamasurg.2017.0904
- Ban, K. A., Minei, J. P., Laronga, C., Harbrecht, B. G., Jensen, E. H., Fry, D. E., Itani, K. M. F., Dellinger, P. E., Ko, C. Y., & Duane, T. M. (2017). American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 update. Journal of the American College of Surgeons, 224(1), 59–74. https://doi.org/10.1016/j.jamcollsurg.2016.10.029