3M™ Ioban™ CHG Chlorhexidine Gluconate Incise Drape (2% w/w CHG), 8850EZSB, 22 in x 17 in (56 cm x 45 cm), 100/CS
About the product
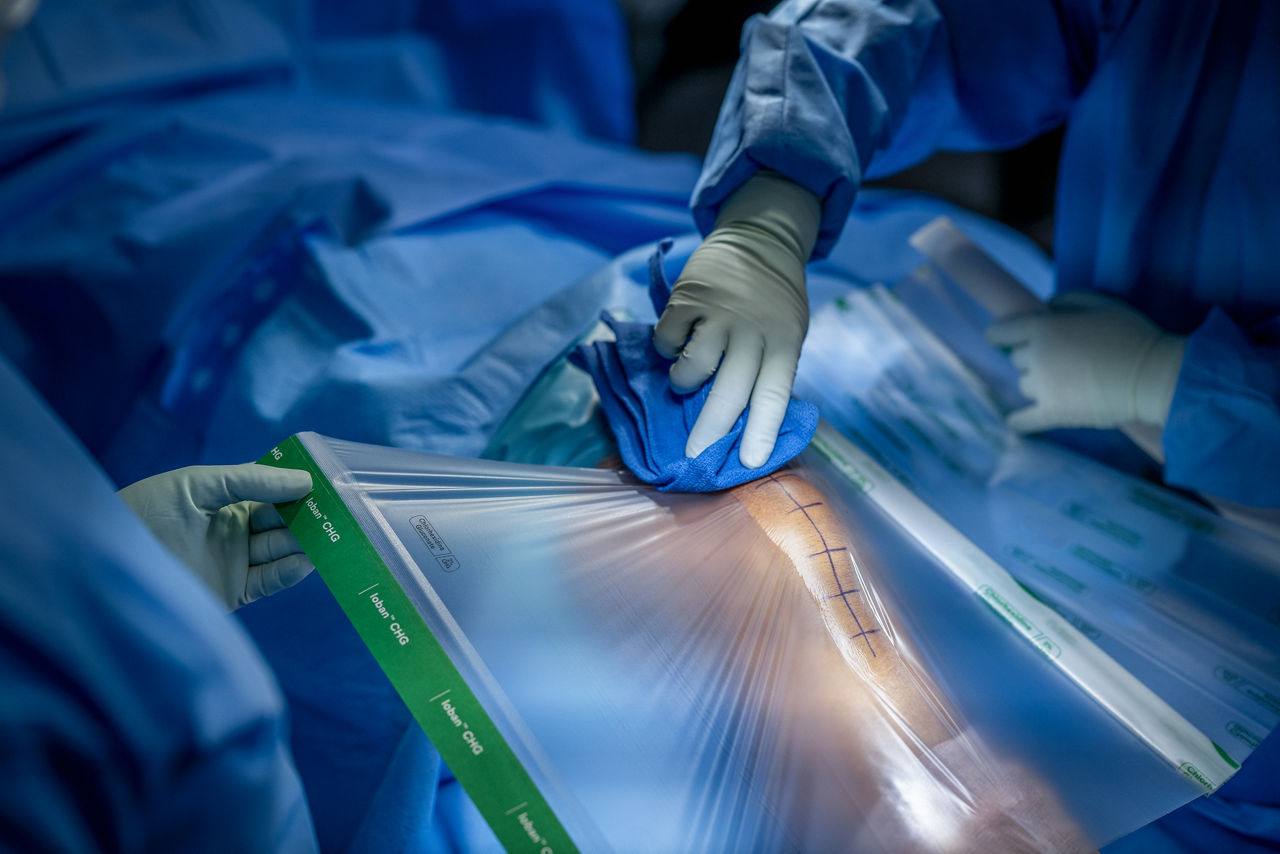
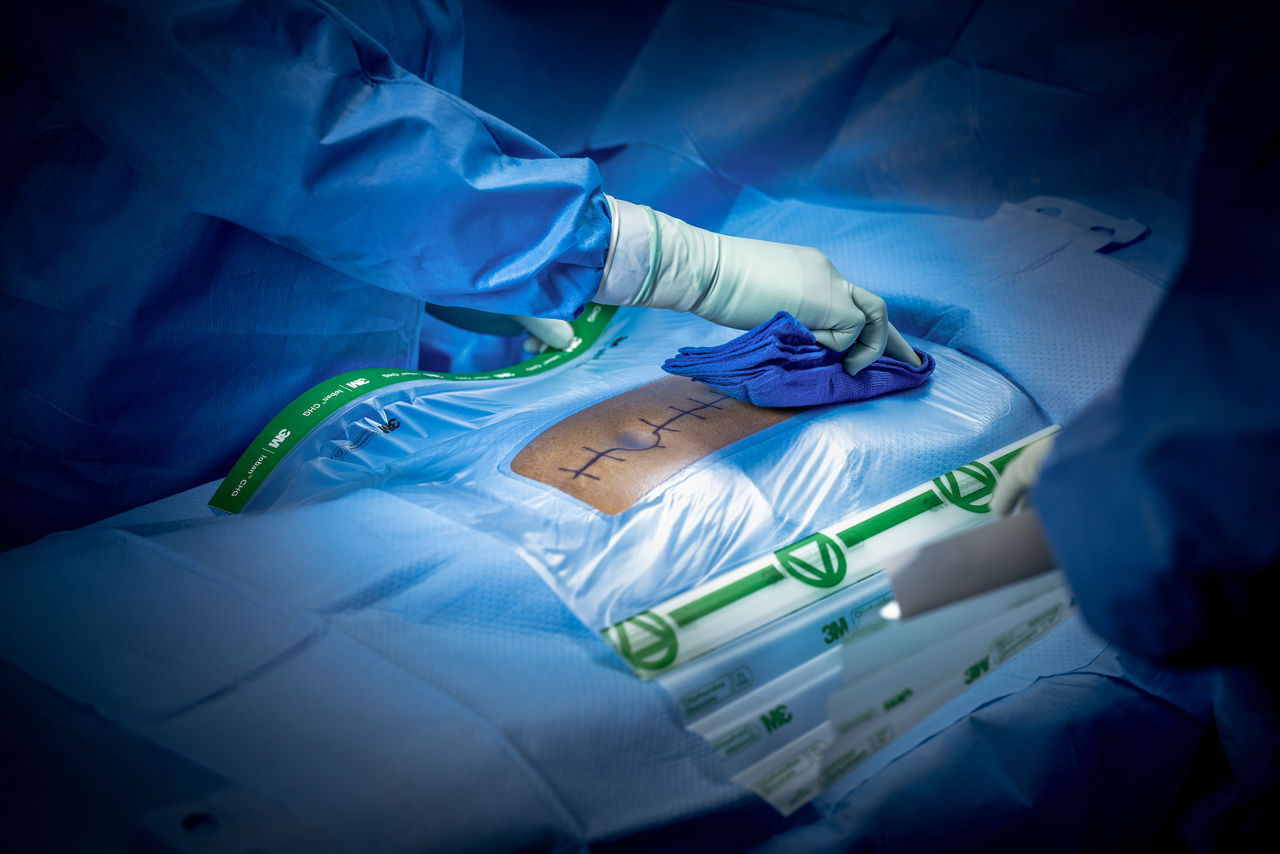
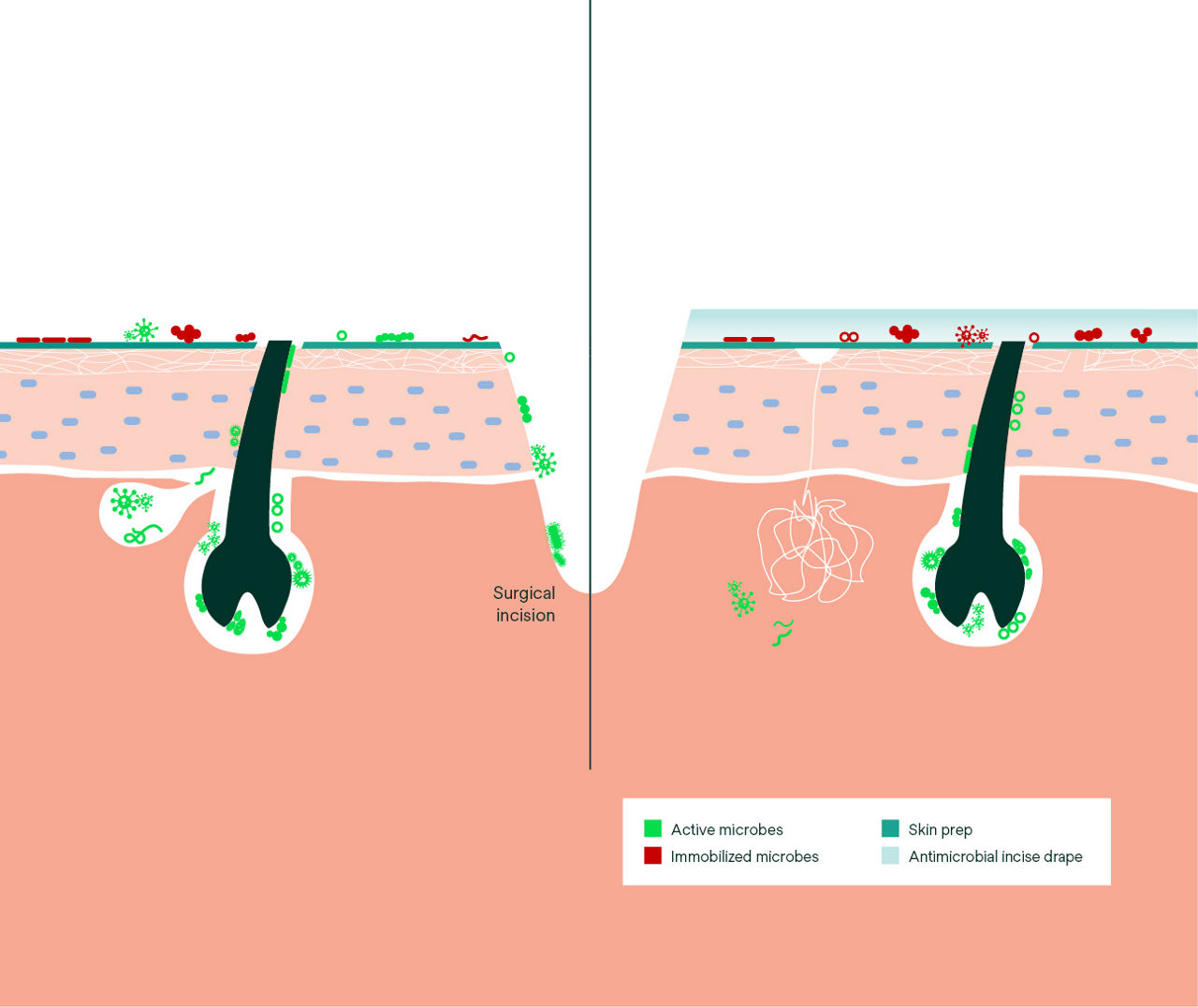
An enhanced innovation in a legacy of proven protection, the Ioban CHG Chlorhexidine Gluconate Incise Drape (contains 2% w/w CHG) creates a sterile operative surface, helping to reduce microbial wound contamination.
Product details
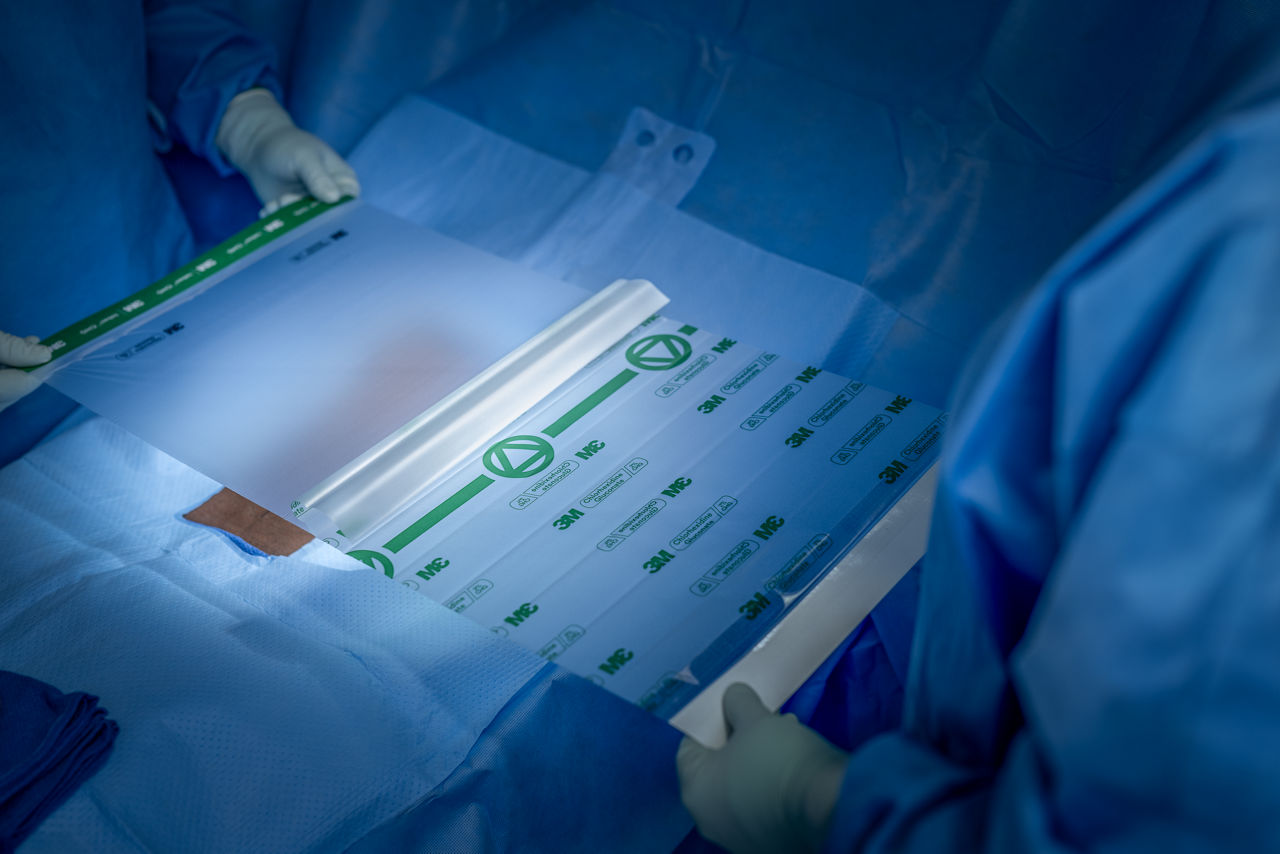
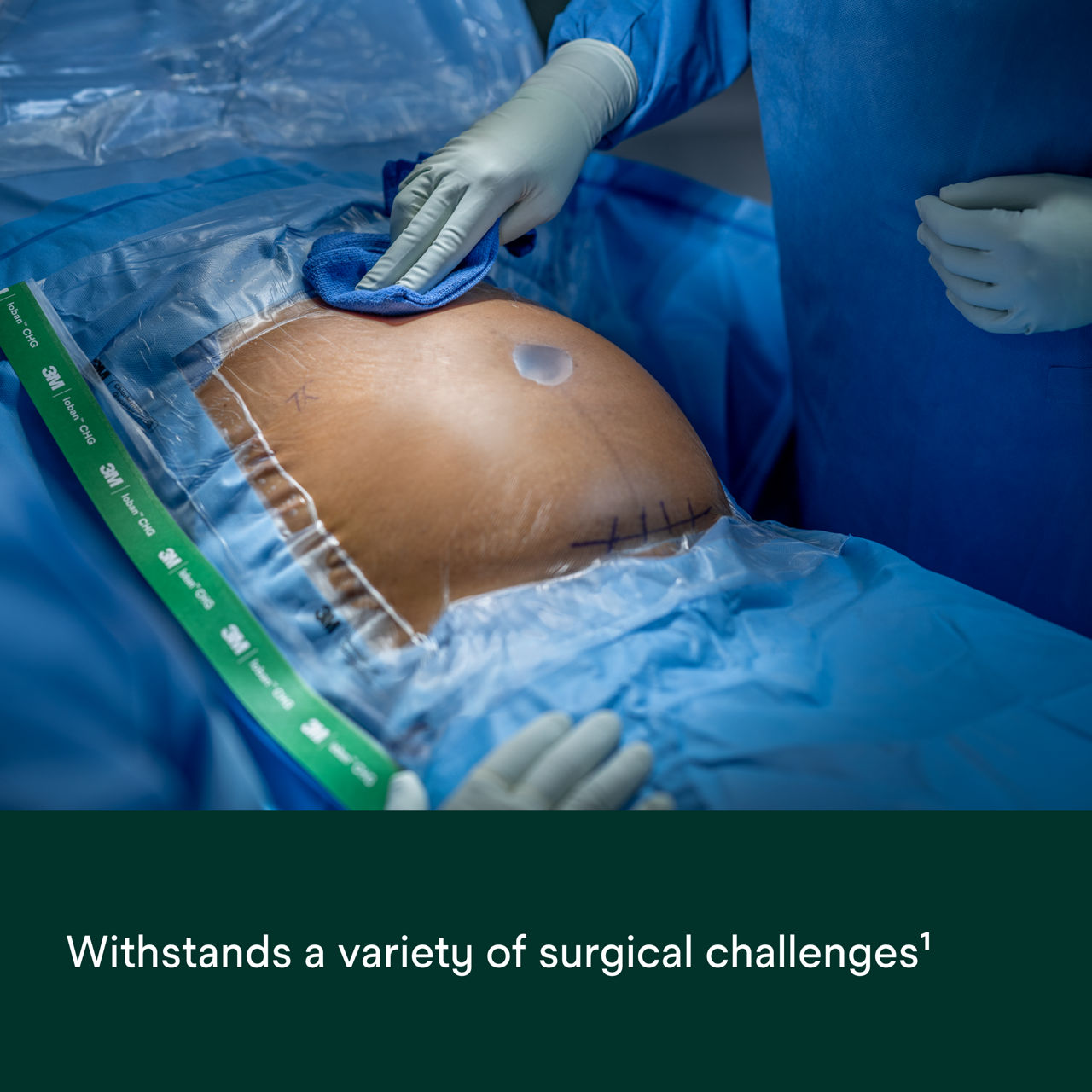
- Strong, durable, breathable, tear-resistant CHG incise drape conforms to body contours and allows for limb manipulation.
- Transparent design allows for easy viewing of the surgical site, including markings and scars.
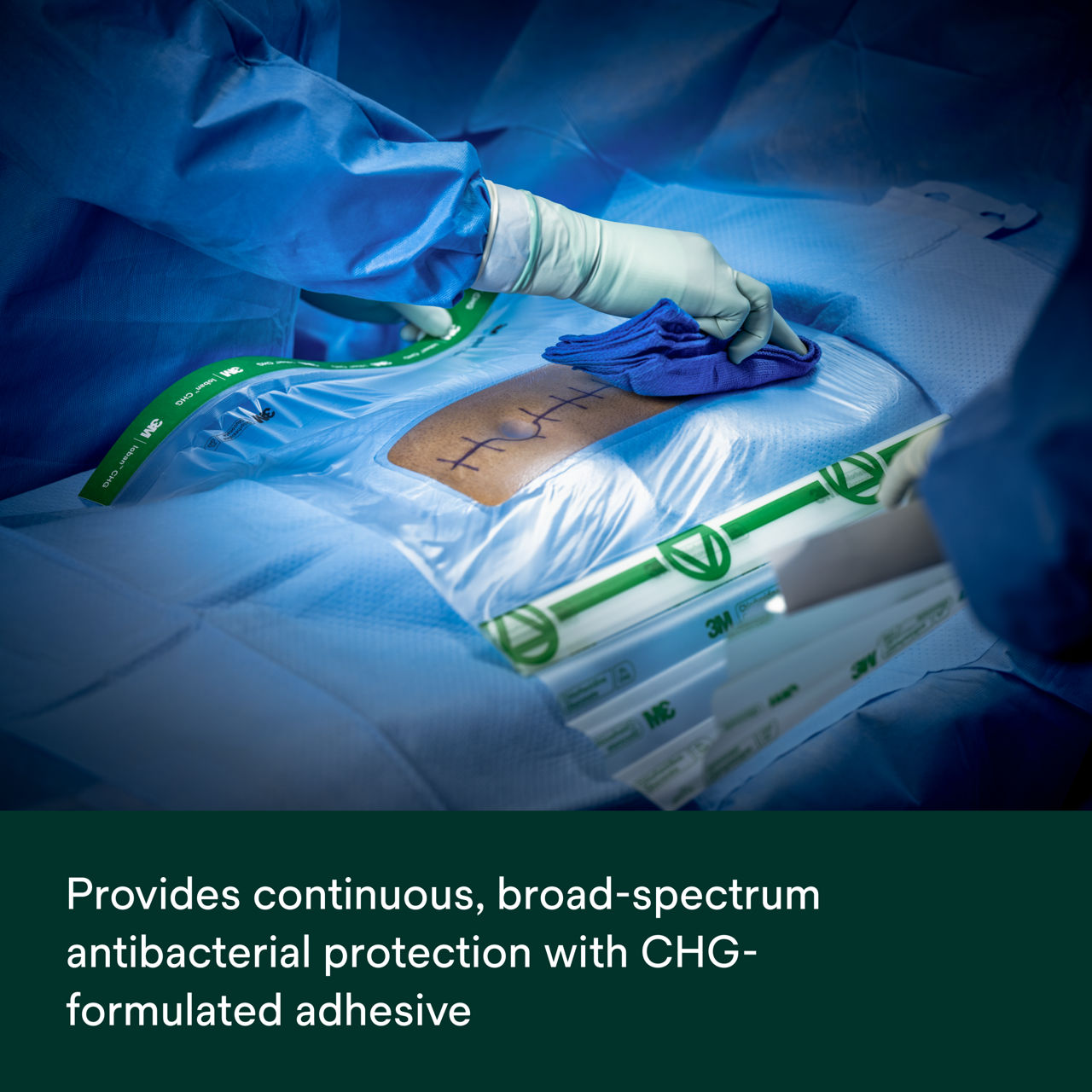
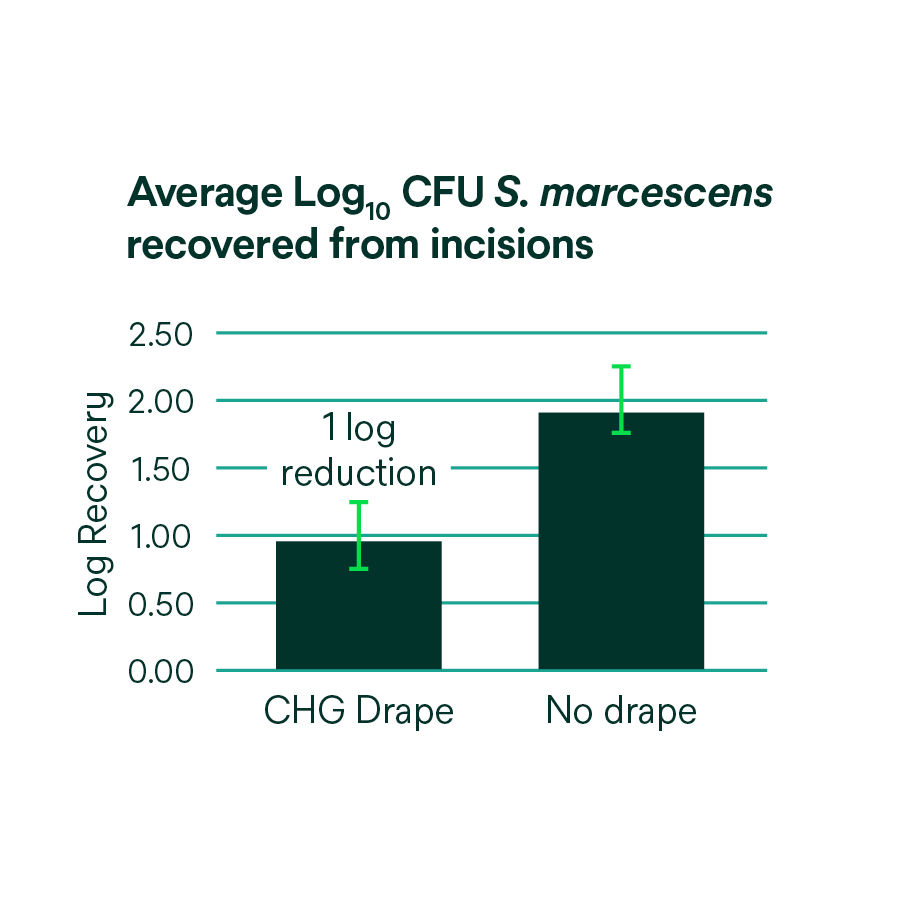
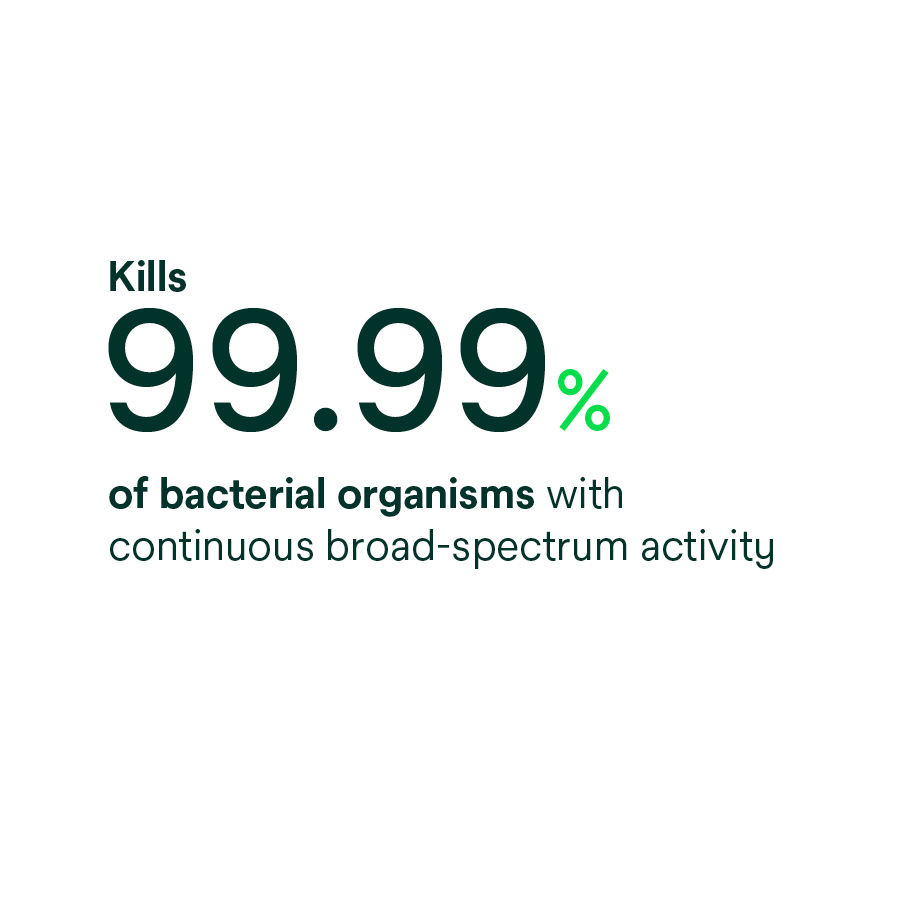
- Provides continuous, broad-spectrum antibacterial activity against a variety of gram-positive and gram-negative bacteria, including antibiotic-resistant bacteria (MRSA, VRE, MRSE) based on in vitro testing.* *In vitro data, clinical significance is unknown.
- Provides a barrier to fluids such as blood and other fluids.
- Protects against prep wash-off by fluids commonly encountered during surgical procedures, including irrigation solutions and blood.
- Skin-friendly incise drape is safe to use on fragile, dry skin and older skin types with minimal skin damage after removal.
- Not made with natural rubber latex.
Suggested Applications
- Open surgical procedures.
Legal
- 1. Solventum internal data on File
- 2. Rhee Y, Palmer LJ, Okamoto K. Differential effects of chlorhexidine skin cleanings methods on residual chlorhexidine skin concentrations and bacterial recovery. Infect Control Hosp Epidemiol. Apr 2018;39(4):405-411.Data on File (Sterile surface).
Product specifications
| Overall Length (Imperial) |
Overall Length (Imperial)
22.05 in |
| Overall Width (Metric) |
Overall Width (Metric)
45 cm |
| Overall Length (Metric) |
Overall Length (Metric)
56 cm |
| Catalog number |
Catalog number
8850EZSB |
| Category name |
Category name
Surgical Drapes |
| Trademark2 |
Trademark2
Ioban™ |
| Overall Width (Imperial) |
Overall Width (Imperial)
17.72 in |