3M™ Tegaderm™ Transparent Film Roll, 16004, 10cm x 10m, 1 Roll/Carton, 4Carton/Case
About the product
Designed to secure medical devices and primary dressings to the skin for up to seven days, the Tegaderm Transparent film roll provides a bacterial1 and viral barrier,* ensuring patient comfort and protection.
*In vitro testing shows that the transparent film provides a viral barrier from viruses 27 nm in diameter or larger while the dressing remains intact without leakage.
Product details
This non-sterile Tegaderm™ Transparent film dressing features a transparent film and adhesive, allowing for precise dressing placement and continu-ous observation of the wound site. It is easy to apply and remove with gloved hands and your patients can wear it while bathing or showering,* allowing them to maintain their regular routine. Plus, you can use it with our other medical products, including the Cavilon™ No Sting Barrier Film and Cavilon™ Durable Barrier Cream, as clinically appropriate.
*Recommended use of waterproof cover over dressing and I.V. site when showering
- Provides a waterproof, sterile barrier to external contaminants including liquids, bacteria1 and viruses*. *In vitro testing shows that the transparent film provides a viral barrier from viruses 27 nm in diameter or larger while the dressing remains intact without leakage
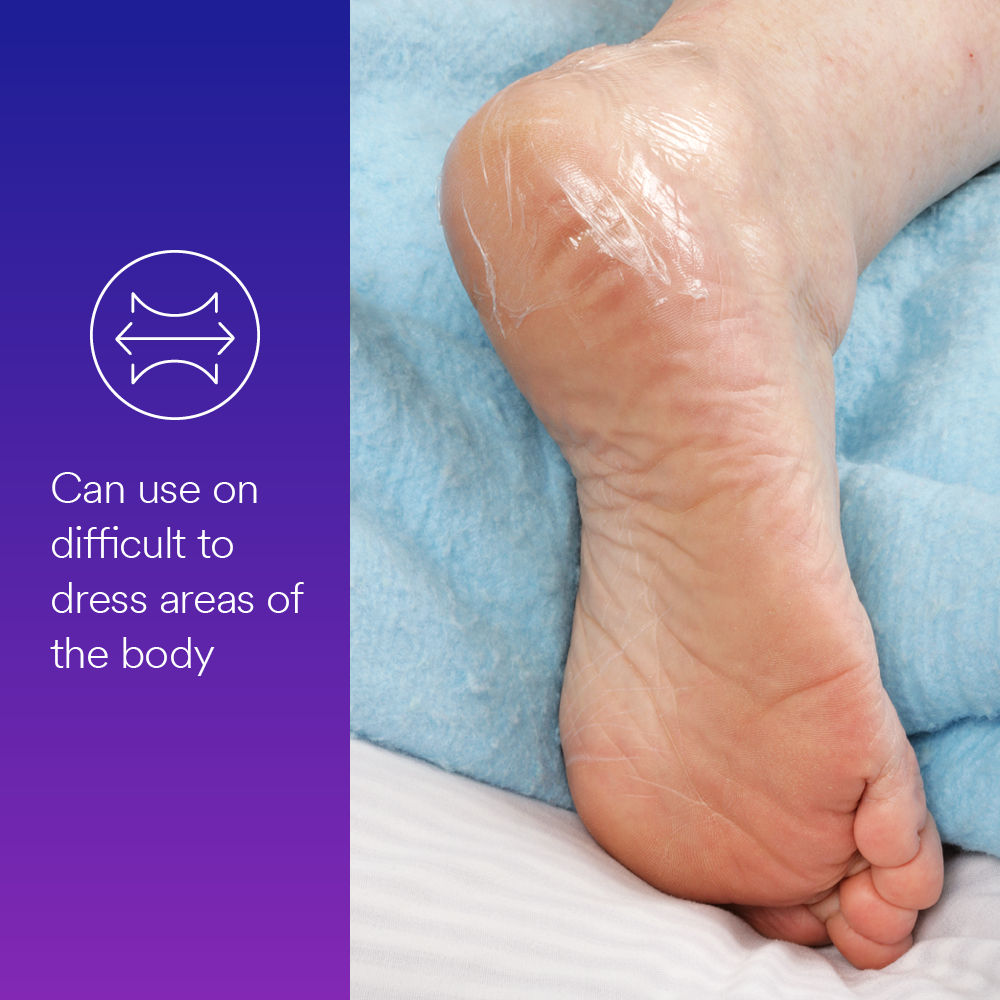
- Can be cut and customized to desired length and shape
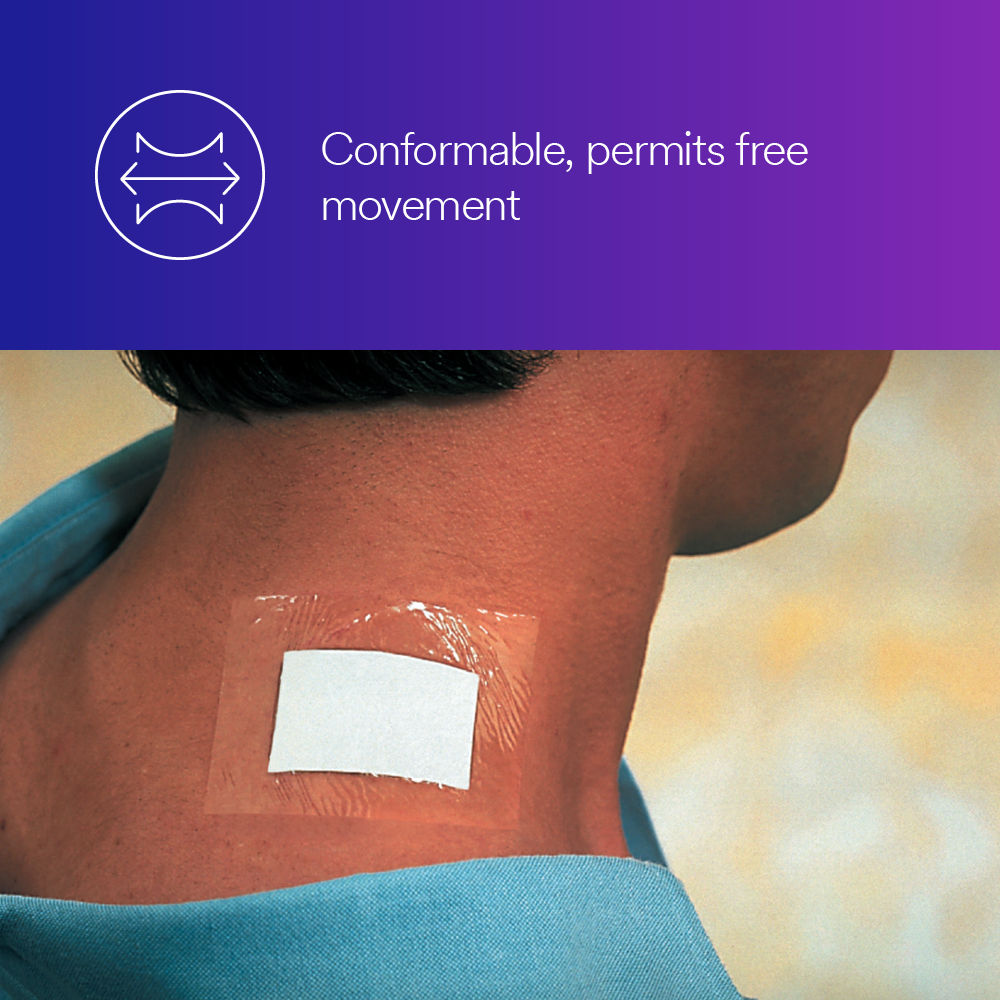
- Breathable, conformable dressing flexes with skin for patient comfort.
- Gentle adhesive has a low dermatitis potential, helping to minimize skin irritation.
- Radiologically transparent — Tegaderm products can be safely left on patient’s skin during radiotherapy treatments and dressing does not act as bolus materi-al, with or without Cavilon™ No Sting Barrier Film.
- May be worn for up to seven days.
- Visually transparent film and adhesive allow for easy dressing placement and removal with gloved hands.
- Can be used with other medical products, including Cavilon™ No Sting Barrier Film and Cavilon™ Durable Barrier Cream, as clinically appropriate.
Suggested Applications
- Holds primary wound dressings and/or non-sterile medical devices securely to skin
- Secondary dressing over alginates, hydrogels, and gauze or for other applications where non-sterile tape would be used
- Skin protection
- Protect intact skin during negative pressure wound therapy, e.g. periwound skin
- Protective eye covering, e.g. to keep eyes closed during surgery and help maintain eye moisture for patients with a corneal abrasion.
- Protect temporary skin markings used for radiation therapy
- Post tattoo application and removal
- Secondary dressing cover while showering.
Legal
1. O'Grady, N et al. (2011). Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011. Retrieved
from http://www.cdc.gov/hicpac/BSI/BSF guidelines2011.htm.
Product specifications
| Brand |
Brand
Tegaderm™ |
| Overall Length (Imperial) |
Overall Length (Imperial)
393.7 in |
| Overall Width (Metric) |
Overall Width (Metric)
10 cm |
| Overall Length (Metric) |
Overall Length (Metric)
10 m |
| Waterproof |
Waterproof
true |
| Dressing Type |
Dressing Type
Film |
| Category name |
Category name
Transparent Film Dressings |
| Overall Width (Imperial) |
Overall Width (Imperial)
3.94 in |
| Border |
Border
false |