3M™ Tegaderm™ Transparent Film Dressings with Border 1635, 50Dressings/Carton, 4 Cartons/Case
About the product
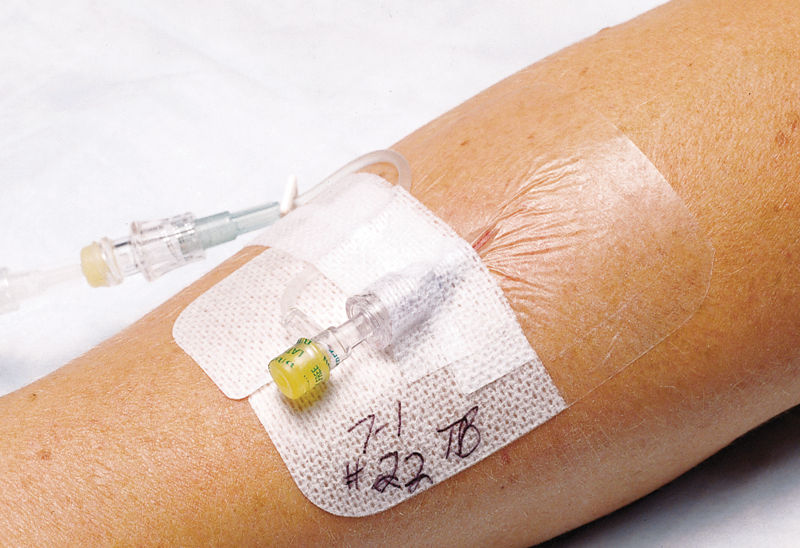
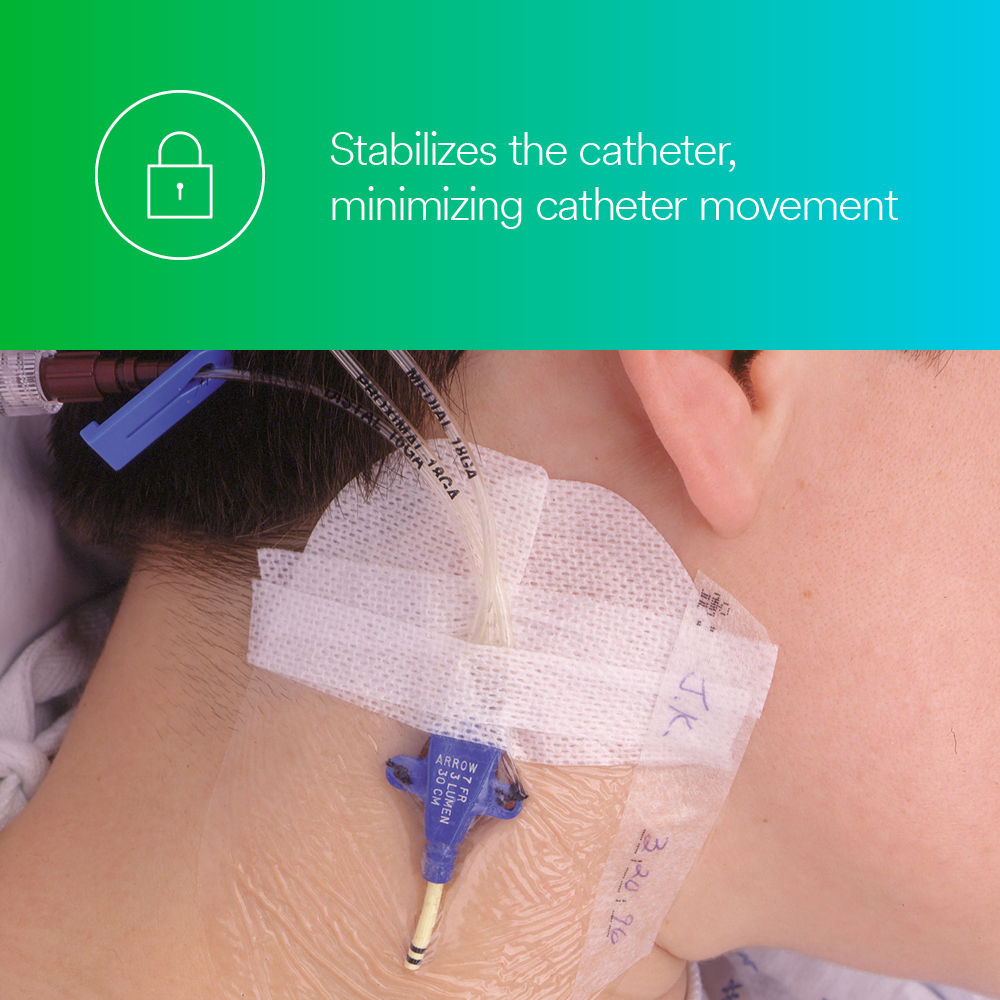
Designed to protect I.V. catheter sites and wounds, the Tegaderm I.V. Transparent Film Dressing with Border secures devices to the skin while maintain-ing a moist wound healing environment and providing a bacterial1 and viral barrier.*
*In vitro testing shows that the Tegaderm Dressing provides a viral barrier from viruses 27 nm in diameter or larger while the dressing remains intact without leakage.
Product details
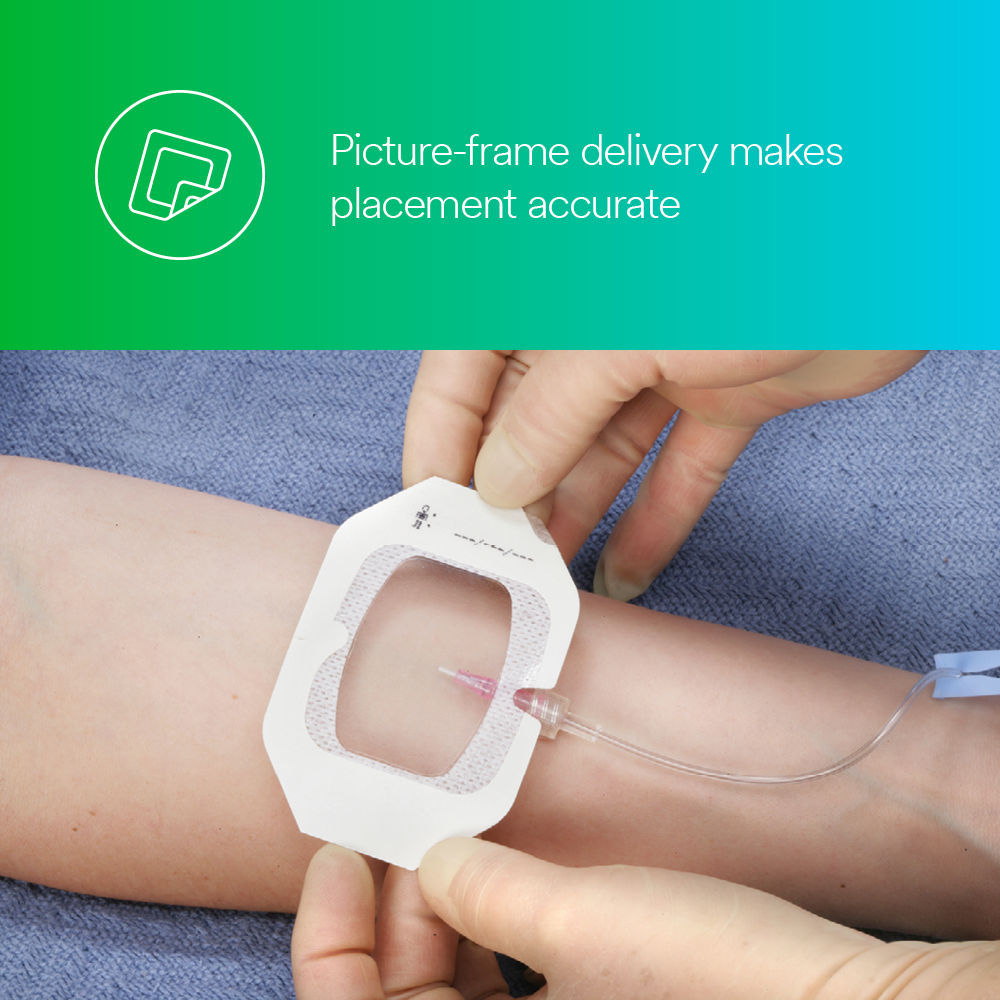
This sterile, breathable, waterproof, vapor-permeable dressing features a transparent film and adhesive, allowing for precise placement and continuous ob-servation of the insertion site. It is easy to apply and remove and your patients can wear it while bathing or showering,* allowing them to maintain their reg-ular routine. Plus, you can use it with our other medical products, including Cavilon™ No Sting Barrier Film.
*Recommended use of waterproof cover over dressing and IV site when showering
- Transparent dressing allows for continuous visibility of the I.V. insertion site.
- Breathable, conformable dressing flexes with skin for patient comfort.
- Picture-frame delivery makes accurate placement easy.
- Provides a waterproof, sterile barrier to external contaminants, including liquids, bacteria1 and viruses.* *In vitro testing shows that the Tegaderm™ Dressing provides a viral barrier from viruses 27 nm in diameter or larger while the dressing remains intact without leakage.
- Adhesive is gentle to skin.
- Can be safely left on central venous catheters for up to seven days.1
- Not made with natural rubber latex.
Suggested Applications
- To cover and protect I.V. catheter sites.
- Acute and chronic wounds.
- Skin protection.
- Feeding tubes.
- Superficial partial thickness burns.
- Stage I or II pressure ulcers.
- Maintains a moist environment for superficial wound healing, making it a useful dressing for minor abrasions, superficial decubitus ulcers, skin graft donor sites and primarily closed clean surgical wounds.
- May be used as protective eye covering during surgery.
- Post-tattoo application and removal.
Legal
1. O'Grady, N et al. (2011). Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011. Retrieved from http://www.cdc.gov/hicpac/BSI/BSF guidelines2011.htm.
Product specifications
| Brand |
Brand
Tegaderm™ |
| OverallLengthMetric |
OverallLengthMetric
10.5 cm |
| OverallLengthImperial |
OverallLengthImperial
4.13 in |
| Waterproof |
Waterproof
true |
| OverallWidthImperial |
OverallWidthImperial
3.35 in |
| Dressing Type |
Dressing Type
Film |
| OverallWidthMetric |
OverallWidthMetric
8.5 cm |
| Border |
Border
true |
| categoryName |
categoryName
Transparent Film Dressings |