3M™ Ioban™ 2 Antimicrobial Incise Drape, 6650, Sterile, 10 Each/Carton,4 Carton/Case
About the product
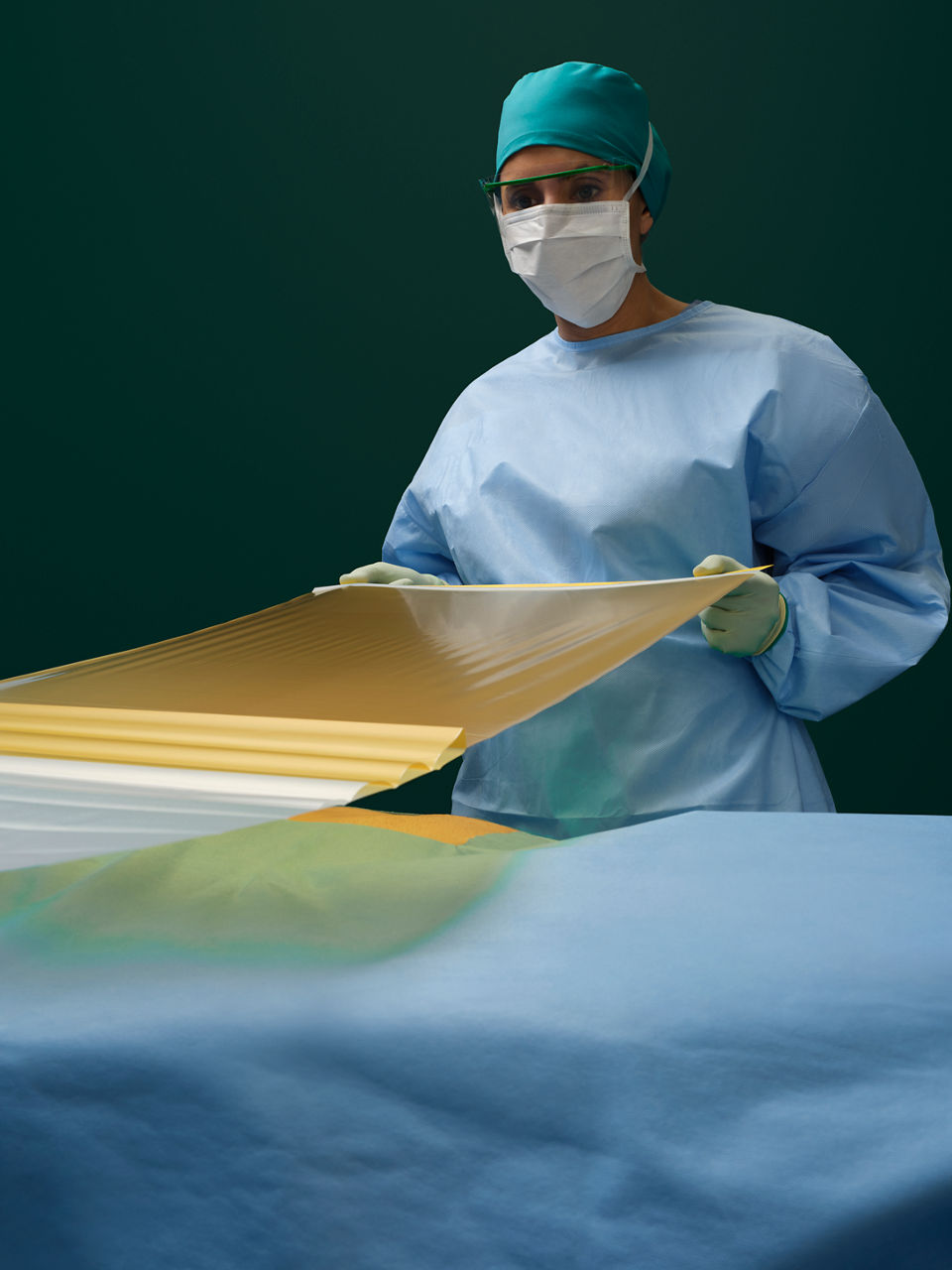
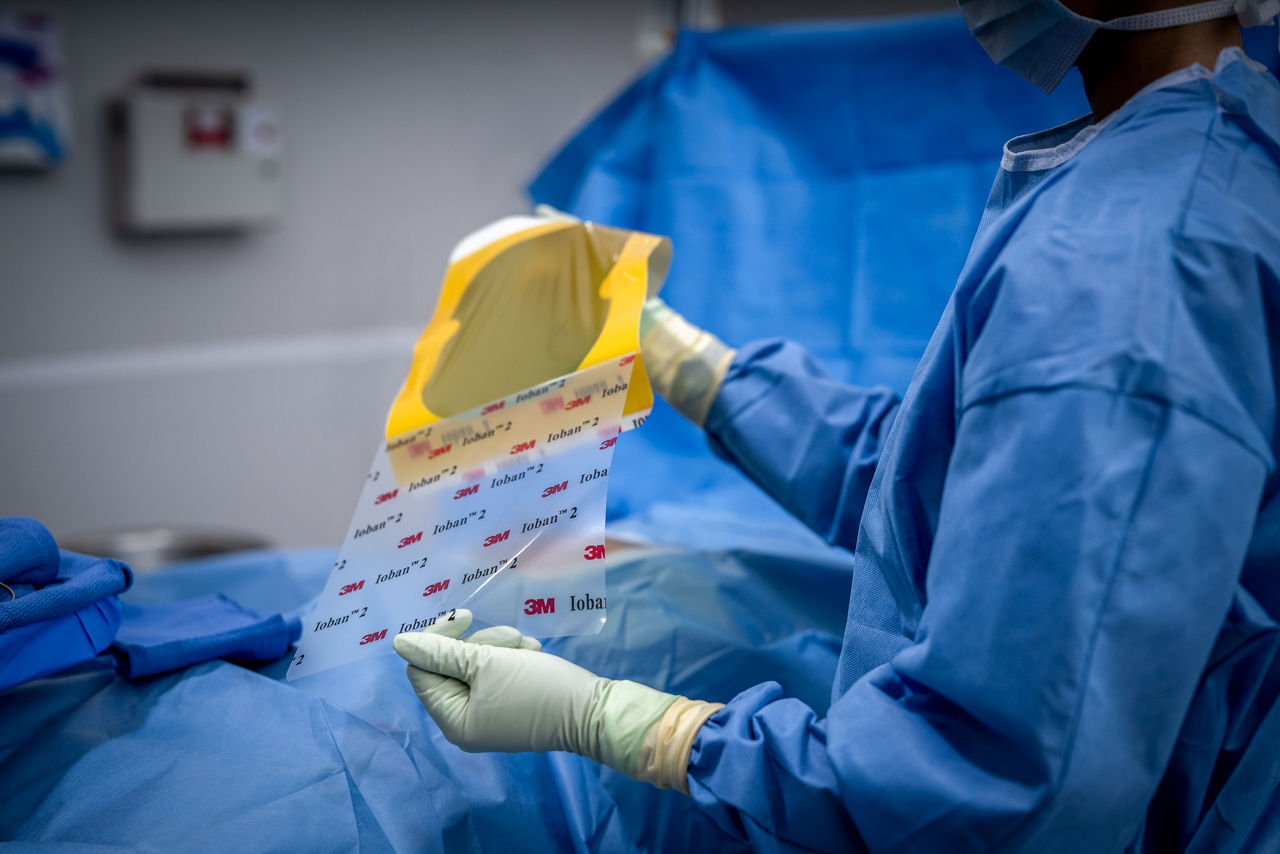
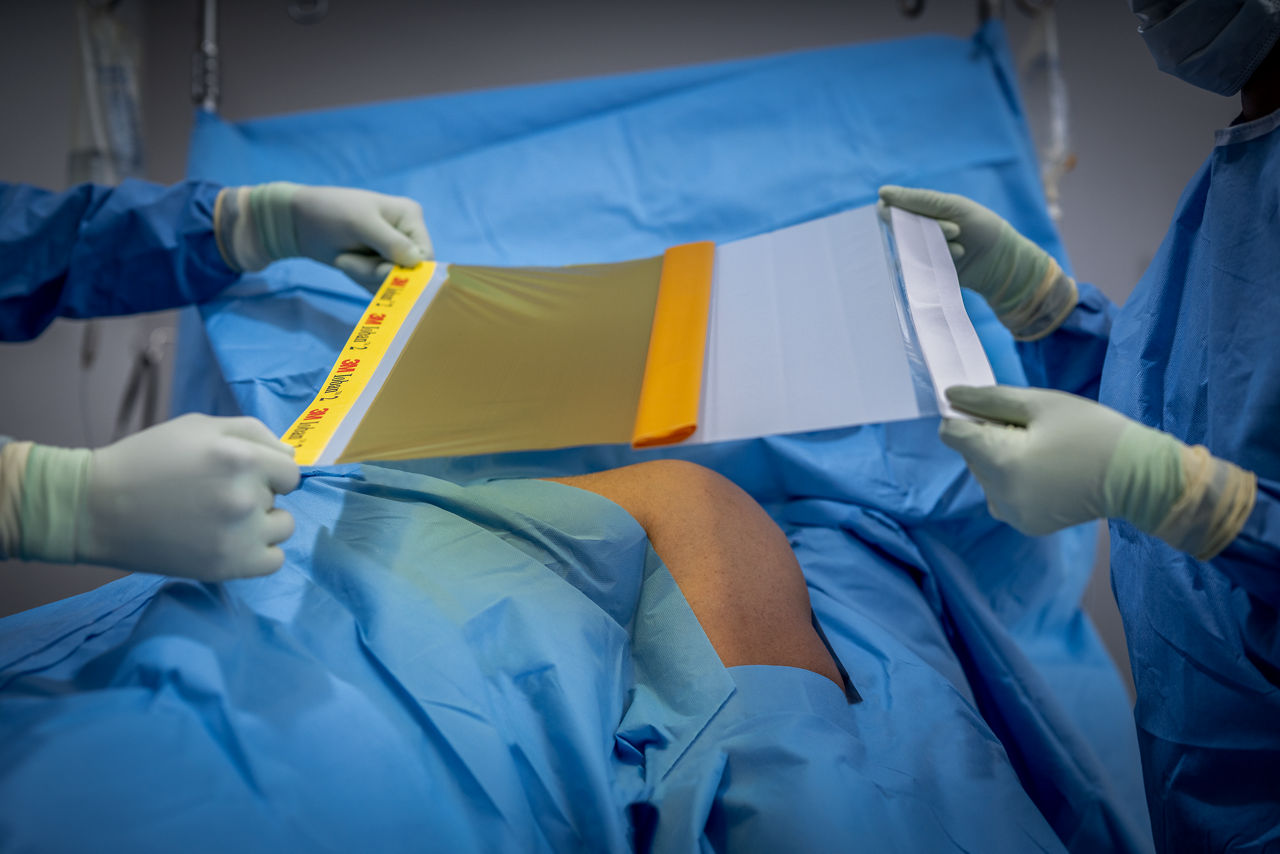
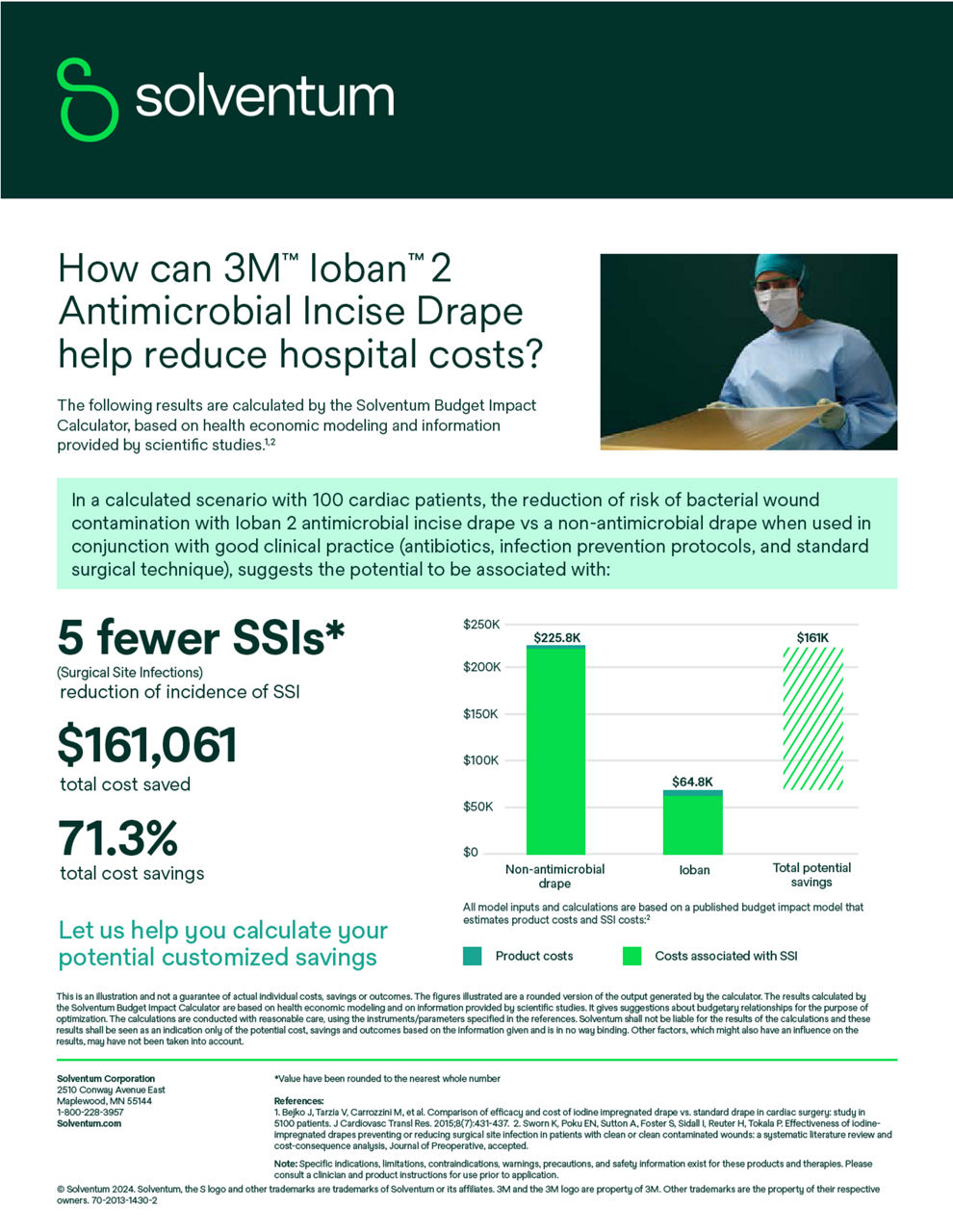
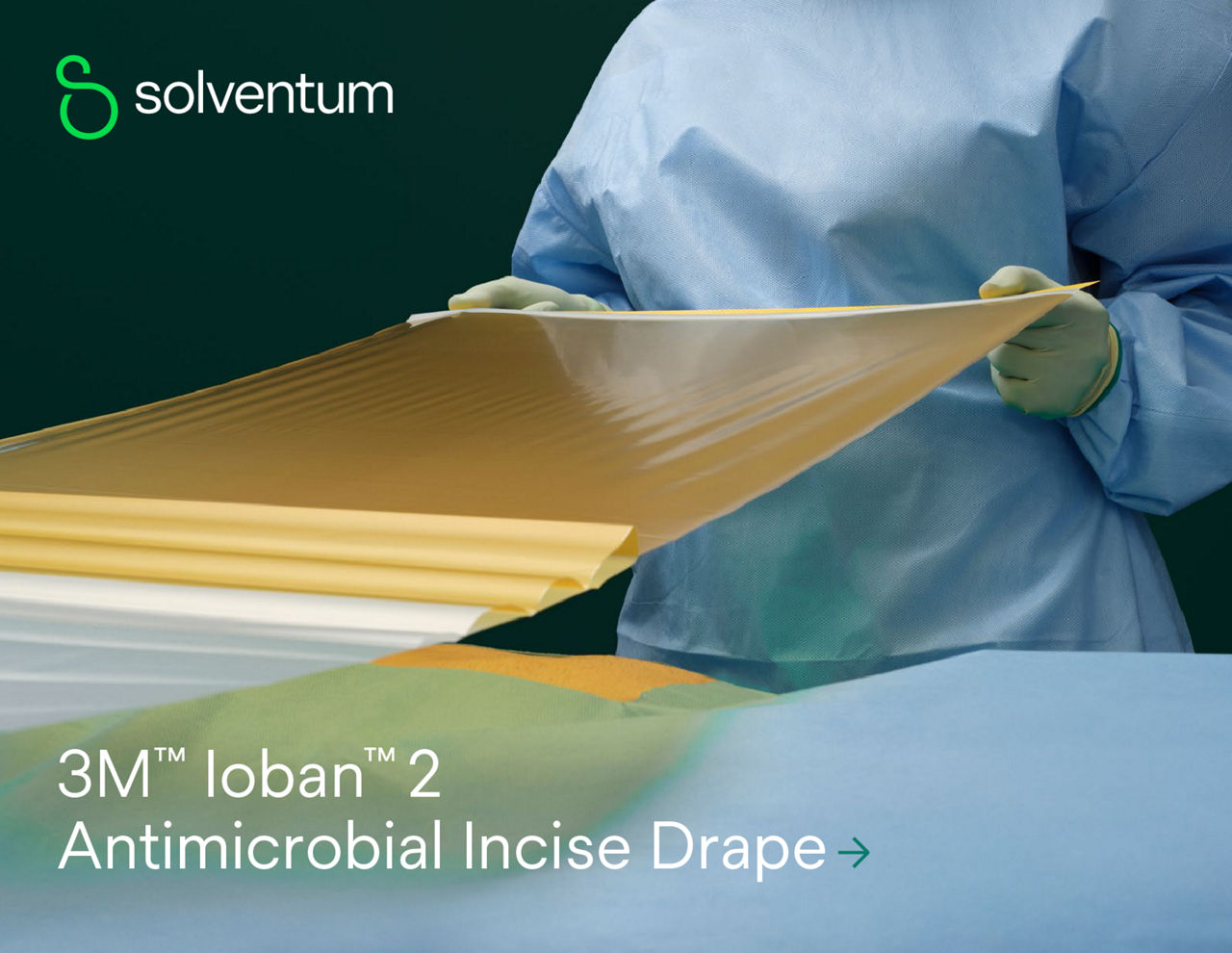
Ioban 2 Antimicrobial Incise Drape provides a powerful barrier to help reduce against microbial wound contamination. To help guard against surgical site infections (SSIs), Ioban 2 Antimicrobial Incise Drape creates an optimized wound incision environment through continuous antimicrobial activity, immobilizing bacteria and conformable adhesion that helps the drape stay in place throughout the surgical procedure.
Product details
- Sterile, breathable, strong, durable, tear-resistant drape with conformable film that adheres to body contours and allows for limb manipulation.
- Drape adhesive provides continuous broad-spectrum antimicrobial activity so the iodine can’t be washed away.
- Clinically proven to help reduce the risk of surgical site contamination and immobilize bacteria on the skin.5,6
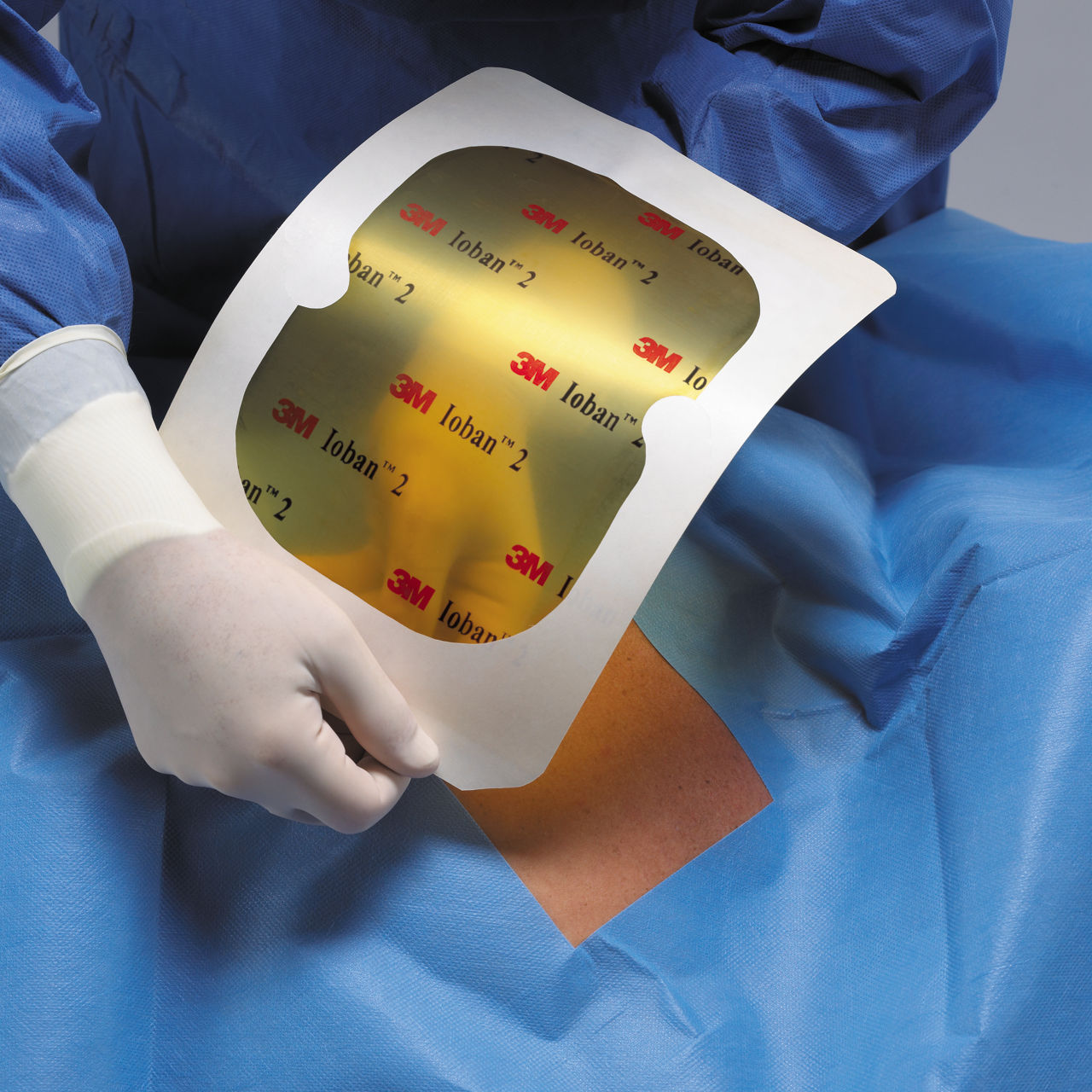
- Creates a sterile surface at the beginning of surgery.
- Adheres to the operative site, eliminating the need for towel clips/clamps and holds the drape in place.
- EZ Delivery design makes the drape easy to apply and remove.
- Demonstrated antimicrobial activity against MRSA in ex vivo human skin samples after six hours of contact.1
- Not made with natural rubber latex.
Suggested Applications
- Incise draping with continuous antimicrobial activity for surgical procedures.
Legal
- 1. Casey AL, Karpanen TJ, Nightingale P, et al. Antimicrobial activity and skin permeation of iodine present in an iodine-impregnated surgical incise drape. J Antimicrob Chemother. 2015, 70:2255-60.
- 2. Bejko J, Tarzia V, Carrozzini M, et al. Comparison of Efficacy and Cost of Iodine Impregnated Drape vs. Standard Drape in Cardiac Surgery: Study in 5100 Patients. J Cardiovasc Transl Res. 2015;8(7):431-437.
- 3. Rezapoor M, Tan TL, Maltenfort MG, et al. Incise Draping Reduces the Rate of Contamination of the Surgical Site During Hip Surgery: A Prospective, Randomized Trial. J Arthroplasty. 2018;33(6):1891-1895.
- 4. Yoshimura Y, Kubo S, Hirohashi K, et al. Plastic iodophor drape during liver surgery operative use of the iodophor-impregnated adhesive drape to prevent wound infection during high risk surgery. World J Surg. 2003, 27:685-8.
- 5. Patrick Arthur Dewan, André M Van Rij, et al. The use of an iodophor-impregnated plastic incise drape in abdominal surgery – a controlled clinical trial. ANZ J Surg. 1987; 57: 859-63.
- 6. Morris French, Harold Eitzen, Merrill Ritter. The plastic surgical adhesive drape: an evaluation of its efficacy as a microbial barrier. Ann Surg. 1976;184:46-50.
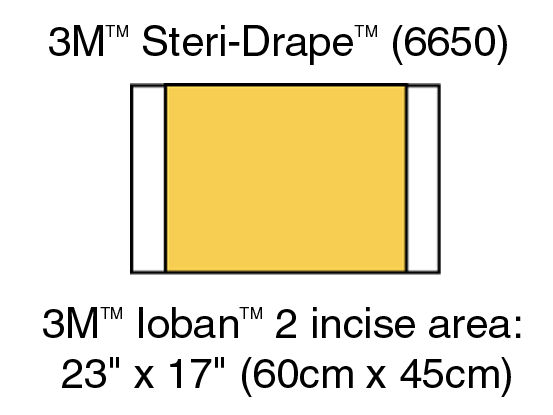
Product specifications
| Antimicrobial |
Antimicrobial
true |
| Brand |
Brand
Ioban™ |
| Overall Length (Imperial) |
Overall Length (Imperial)
25.98 in |
| Overall Width (Metric) |
Overall Width (Metric)
45 cm |
| Overall Length (Metric) |
Overall Length (Metric)
66 cm |
| Drape type |
Drape type
Incise |
| Material |
Material
Plastic |
| Category name |
Category name
Surgical Drapes |
| Overall Width (Imperial) |
Overall Width (Imperial)
17.72 in |