3M™ Dermatac™ Drape
About the product
Provide the ideal balance of wound healing support for patients receiving negative pressure wound therapy (NPWT) with the Dermatac Drape, the first silicone-acrylic hybrid drape designed for use with V.A.C.® Therapy. Combining the benefits of forgiving silicone with the strength and stability of adhesive acrylic, our skin-friendly drape helps to improve handling, placement and patient comfort.
Product details
V.A.C.® Therapy patients will feel more comfortable during their wound healing process. Dermatac Drape utilizes a low-tack adhesive technology that supports reaches across the care team: from physician to nurse to patient.
The first hybrid drape to combine skin-friendly silicone with adhesive acrylic. A precise technology that allows it to be easily applied and repositioned during application without losing adhesion to maintain a strong seal, yet gentle enough to help minimize the pain of dressing removal.
- Easy-to-apply silicone-acrylic drape conforms to different anatomical locations and can be repositioned after initial placement.
- Maintains a highly effective seal for negative pressure wound therapy (NPWT).
- Supports wound healing and patient comfort with skin-friendly removal.
- Saves time at dressing changes vs. standard drapes.1
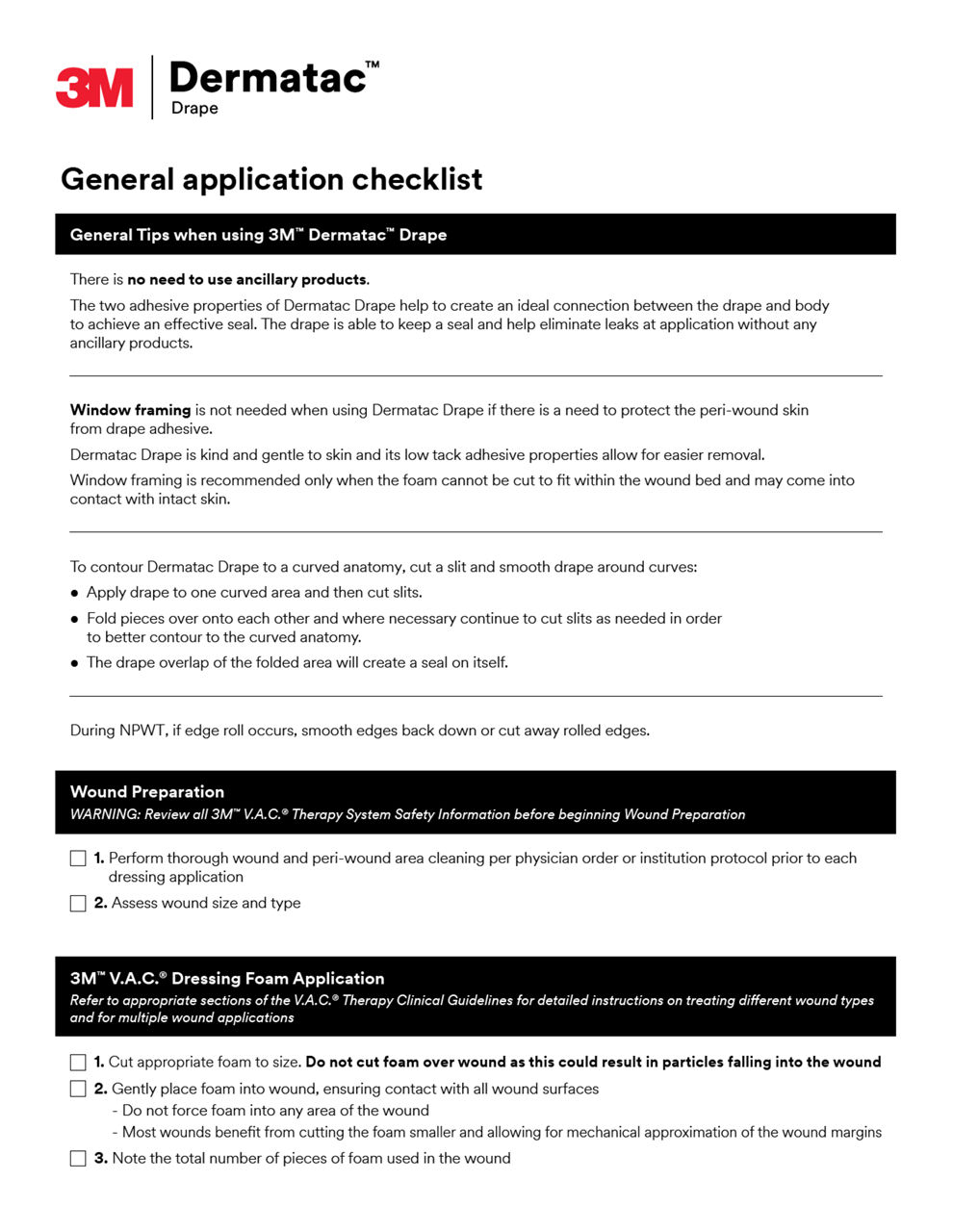
- No need for ancillary products to help with seal.2†
- Reduce dressing application time by 33% compared to the standard V.A.C.® Drape.4,5
- Use the Dermatac Drape with any of the dressings in the V.A.C. portfolio.
Suggested Applications
- Dermatac Drape is an accessory to the following integrated wound management systems for use in acute, extended and home care settings and other professional healthcare environments where product use is conducted by or under the supervision of a qualified healthcare professional: ActiV.A.C.™ Therapy Unit, the V.A.C.® FREEDOM™ Therapy Unit, the V.A.C.® Ulta Therapy Unit, the V.A.C.® RX4 Therapy Unit and the V.A.C.® Simplicity Therapy Unit.
- Designed for use on open wound types, including chronic, acute, subacute and dehisced wounds, partial thickness burns and ulcers (such as diabetic, pressure or venous insufficiency), flaps and grafts.
- Compatible with Granufoam™ Dressing, V.A.C.® Simplace™ Ex Dressing, V.A.C.® Simplace™ Dressing, V.A.C.® Granufoam™ Bridge Dressing, V.A.C.® Granufoam™ Bridge XG Dressing, V.A.C.® Granufoam™ Round Dressing, V.A.C.® Granufoam™ Thin Dressing, V.A.C.® Granufoam™ Silver Dressings and V.A.C.® Whitefoam™ Dressings.
Legal
- †A six case series with anatomically challenging wounds using Dermatac Drape. By the second or third dressing change, ancillary products to help maintain a negative pressure seal were discontinued.
- 1Fernández LG, Matthews MR, Benton C, et al. Use of a novel silicone acrylic drape with negative pressure wound therapy in anatomically challenging wounds. Int Wound J. 2020;17(6):1829-18344.
- 3KCI. Summative User Interface Evaluation Report. March 20, 2018.
- 4ICG. DERMATAC Opportunity Assessment: Qualitative & Quantitative Market Research Final Report. Oct 8, 2015.
- 53M. Summative User Interface Evaluation Report. March 20, 2018.
- 6ICG. Dermatac Opportunity Assessment: Qualitative & Quantitative Market Research Final Report. October 8, 2015. REF-01638.
Product specifications
| Brand |
Brand
Dermatac™ |
| Product Color |
Product Color
Black |
| Category name |
Category name
NPWT Dressings |