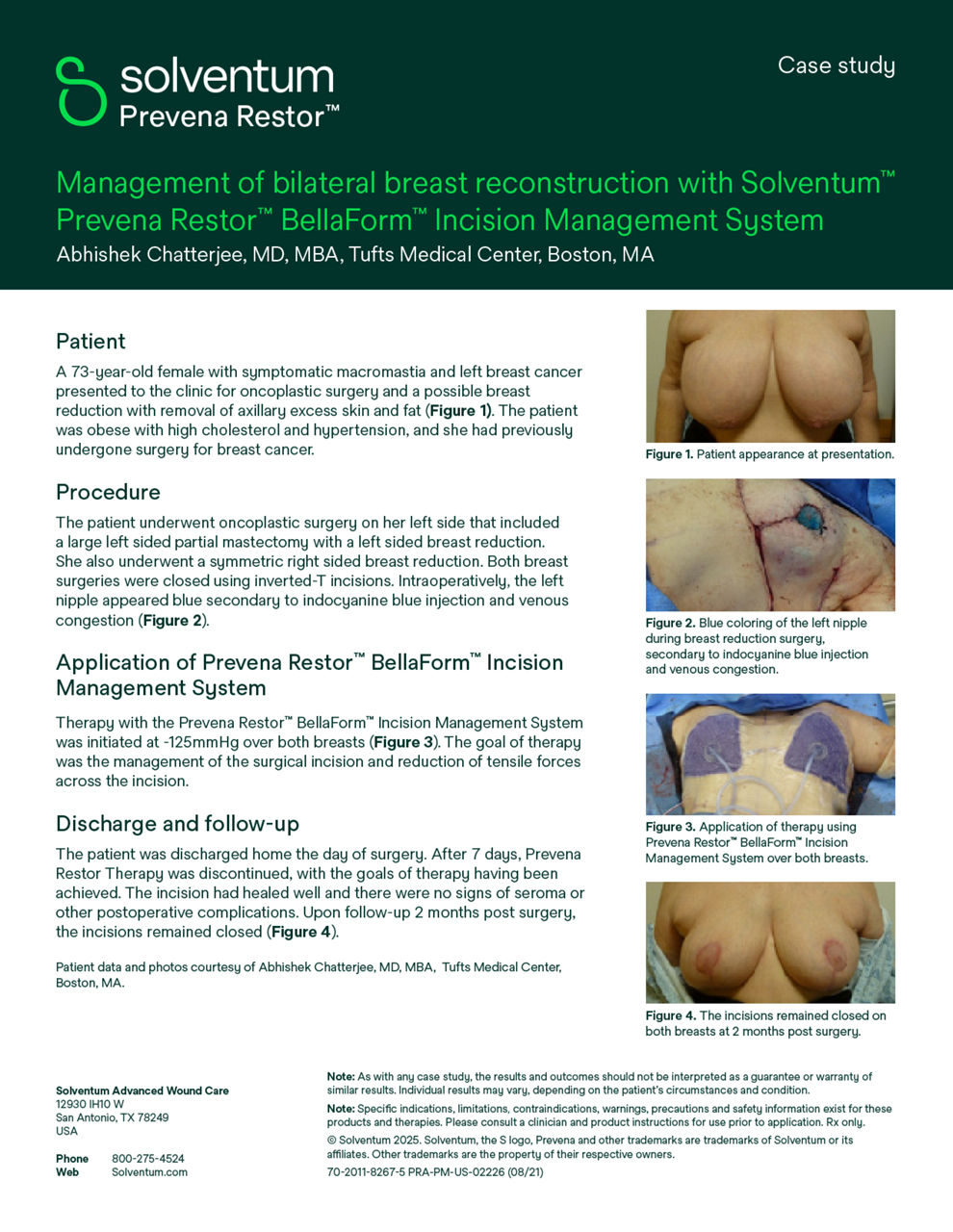
Solventum Prevena Therapy for plastic surgery
Enhanced plastic surgery outcomes with Prevena Therapy
A peer-reviewed meta-analysis of 16 studies for various plastic surgery procedures demonstrated Solventum™ Prevena™ Therapy helped significantly reduce the risk of various surgical site complications (SSCs) while helping to improve health economic outcomes compared to standard-of-care dressings.1
† Calculation(s) are derived based on the relative patient group incidence rate reporting in this study. Statistically significant (p<0.05).
*Relative risk reduction
**The use of Prevena Therapy for the reduction in the incidence of dehiscence has not been reviewed by the U.S. FDA.
*The effectiveness of Prevena Therapy in reducing the incidence of SSIs and seroma in all surgical procedures and populations has not been demonstrated. See full indications for use and limitations at eifu.solventum.com
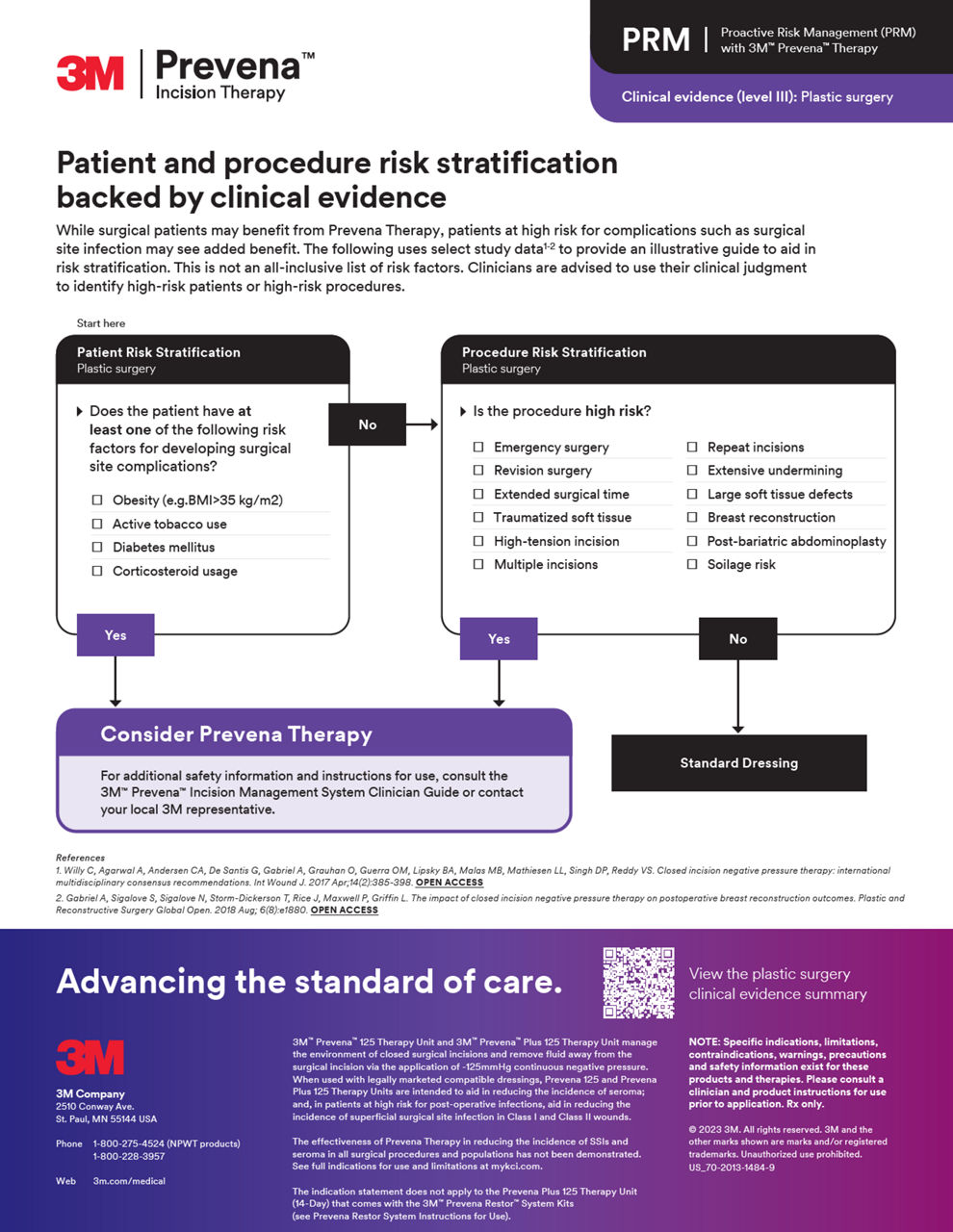
Proactive risk management (PRM)
Access comprehensive resources to implement PRM into your practice with Prevena Therapy, helping elevate your standard of patient care with proven postoperative benefits.
Prevena Therapy video resources
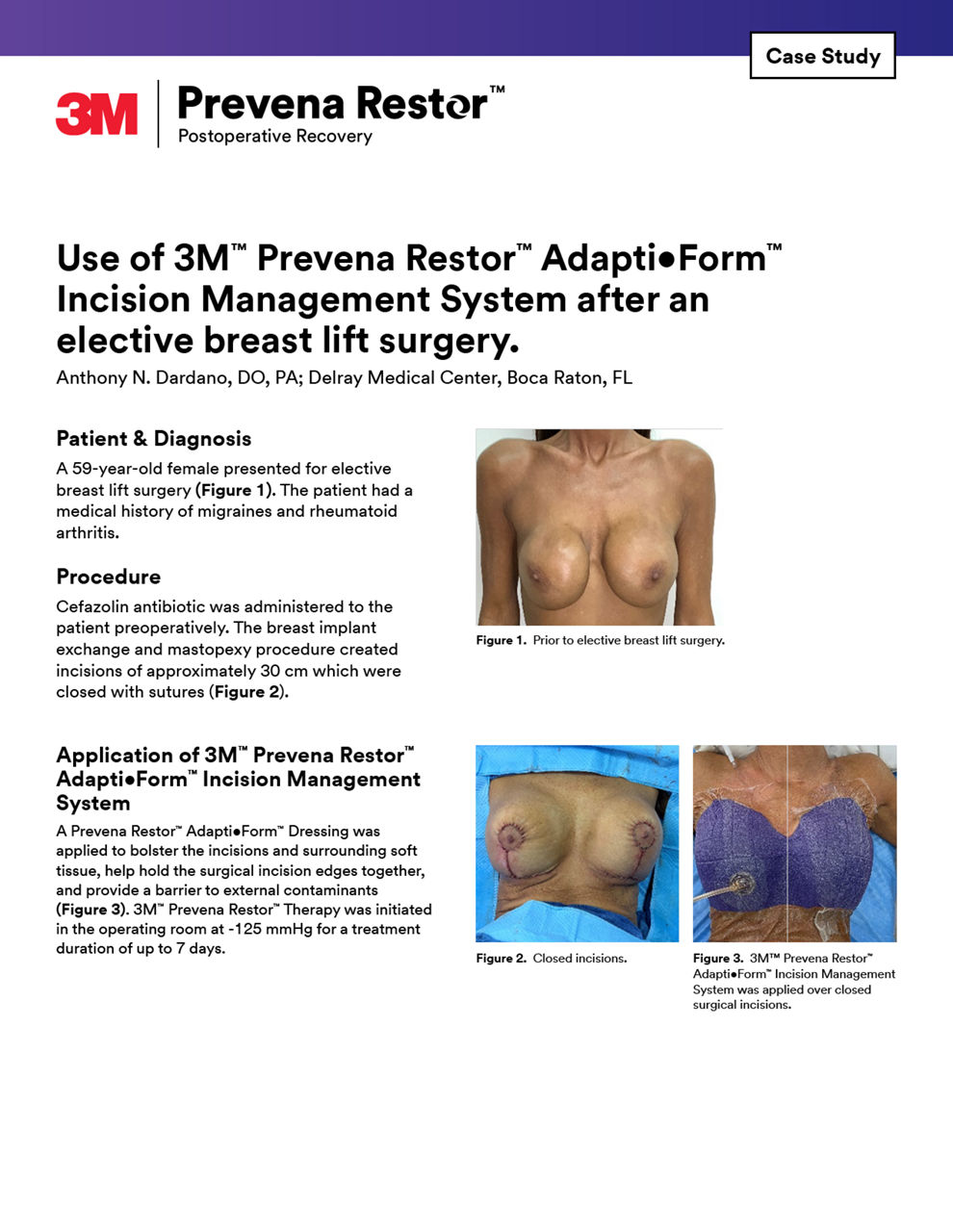
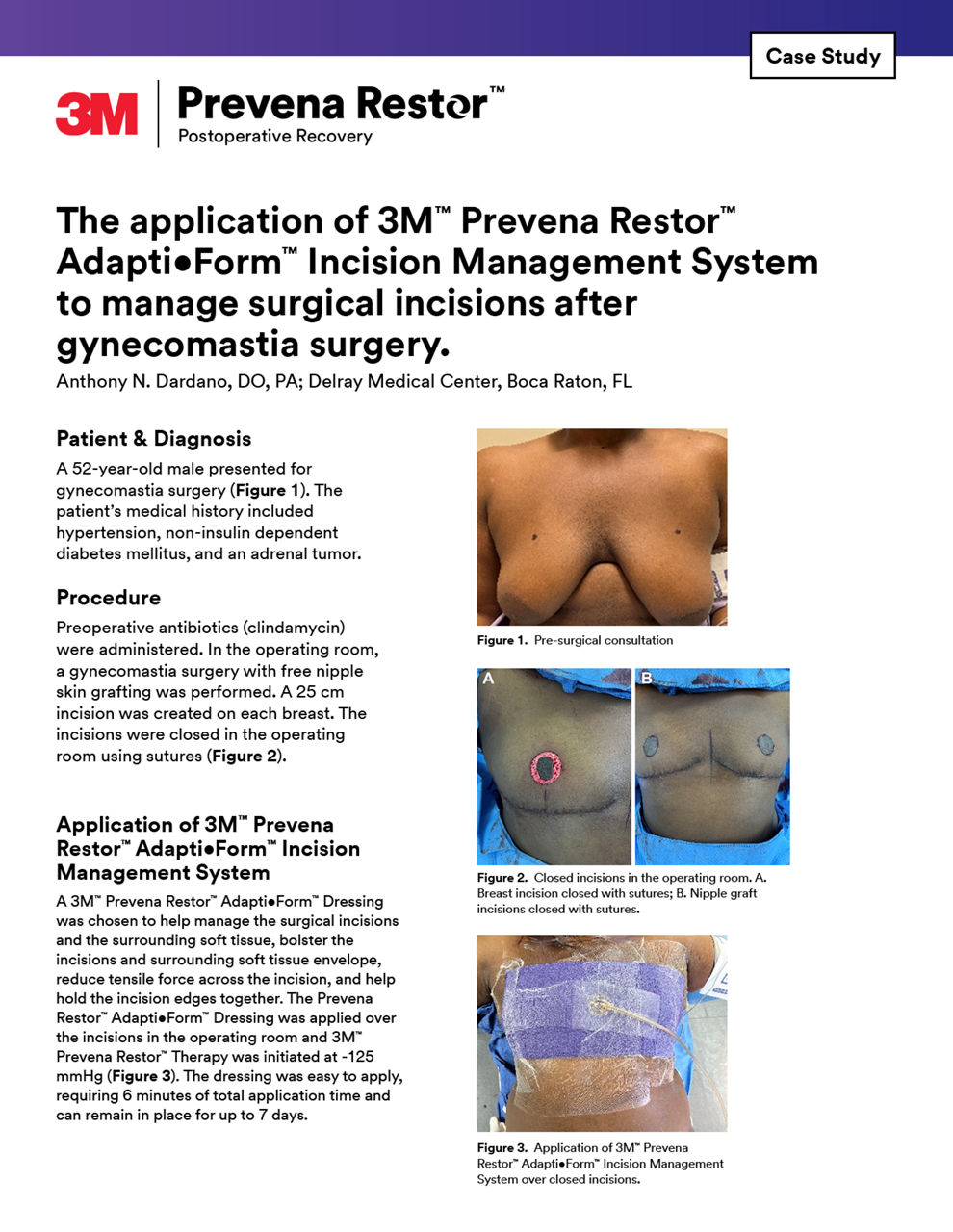
Case studies
Explore our curated selection of case studies, featuring esteemed surgeons and experts that showcase the efficacy of Prevena Therapy in various surgical scenarios.
Explore more
Product information
Explore detailed resources including brochures, coding sheets and other informational resources offering comprehensive insights into Prevena Therapy for plastic surgery.
Abstracts
Discover abstracts from prominent research papers on topics related to Prevena Therapy, offering valuable scientific findings and perspectives.
- Incisional negative pressure therapy reduces complications and costs in pressure ulcer reconstruction
- Preliminary result with incisional negative pressure wound therapy and pectoralis major muscle flap for median sternotomy wound infection in a high-risk patient population
- Economic analysis based on the use of closed-incision negative-pressure therapy after postoperative breast reconstruction
- The impact of closed incision negative pressure therapy on postoperative breast reconstruction outcomes
NOTE:
Specific indications, limitations, contraindications, warnings, precautions and safety information exist for these products and therapies. Please consult a clinician and product instructions for use prior to application. Rx only.
Indication(s) For Use / Intended Use: US FDA Cleared: Only for Use in the United States:
Prevena Dressing used with Prevena Therapy Units: PREVENA™ 125 and PREVENA PLUS™ 125 Therapy Units manage the environment of closed surgical incisions and remove fluid away from the surgical incision via the application of -125mmHg continuous negative pressure. When used with legally marketed compatible PREVENA™ dressings for up to seven days, PREVENA™ 125 and PREVENA PLUS™ 125 Therapy Units are intended to aid in reducing the incidence of seroma; and in patients at high risk for post-operative infections, aid in reducing the incidence of superficial surgical site infection in Class I and Class II wounds.
Limitations:
- The device is not intended to treat surgical site infection or seroma.
- Safety and effectiveness in pediatric population (<22 years old) have not been evaluated.
- Safety and effectiveness in Class III (Contaminated) and Class IV (Dirty/Infected) wounds have not been demonstrated. Furthermore, Class IV surgical wounds are not expected to be closed primarily, and the subject device should only be used on closed surgical incisions.
- The device has not been demonstrated to reduce deep incisional and organ space surgical site infections.
- The device has not been demonstrated to be effective in reducing the incidence of surgical site infection and seroma in all surgical procedures and patient populations; therefore, the device may not be recommended for routine use to reduce the incidence of surgical site infection and seroma.
- Please refer to the ‘Summary of Clinical Information’ section for the specific surgical procedures and patient populations included in the clinical studies. Surgeons should continue to follow the ‘Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection’2 and the ‘American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines’3 for best practices in preventing surgical site infection. US FDA Cleared: Dressings/ Systems (Prevena Dressings used with compatible Solventum NPWT units - ActiVAC, Ulta, and RX4) and applicable OUS countries that leverage US Indication: The PREVENA™, PREVENA PLUS™, PREVENA DUO™, and PREVENA RESTOR™ Incision Management Systems are intended to manage the environment of surgical incisions that continue to drain following sutured or stapled closure by maintaining a closed environment and removing exudates via the application of negative pressure wound therapy.
References:
- Gabriel A, Singh D, Silverman RP, Collinsworth A, Bongards C, Griffin L. ePlasty. 2023 Mar 31;23:e22. Closed incision negative pressure therapy versus standard of care over closed plastic surgery incisions in the reduction of surgical site complications: A systematic review and meta-analysis of comparative studies
- Berríos-Torres, S. I., Umscheid, C. A., Bratzler, D. W., Leas, B., Stone, E. C., Kelz, R. R., Reinke, C. E., Morgan, S., Solomkin, J. S., Mazuski, J. E., Dellinger, E. P., Itani, K. M., Berbari, E. F., Segreti, J., Parvizi, J., Blanchard, J., Allen, G., Kluytmans, J. A., Donlan, R., & Schecter, W. P. (2017). Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surgery, 152(8), 784. https://doi.org/10.1001/jamasurg.2017.0904
- Ban, K. A., Minei, J. P., Laronga, C., Harbrecht, B. G., Jensen, E. H., Fry, D. E., Itani, K. M. F., Dellinger, P. E., Ko, C. Y., & Duane, T. M. (2017). American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 update. Journal of the American College of Surgeons, 224(1), 59–74. https://doi.org/10.1016/j.jamcollsurg.2016.10.029