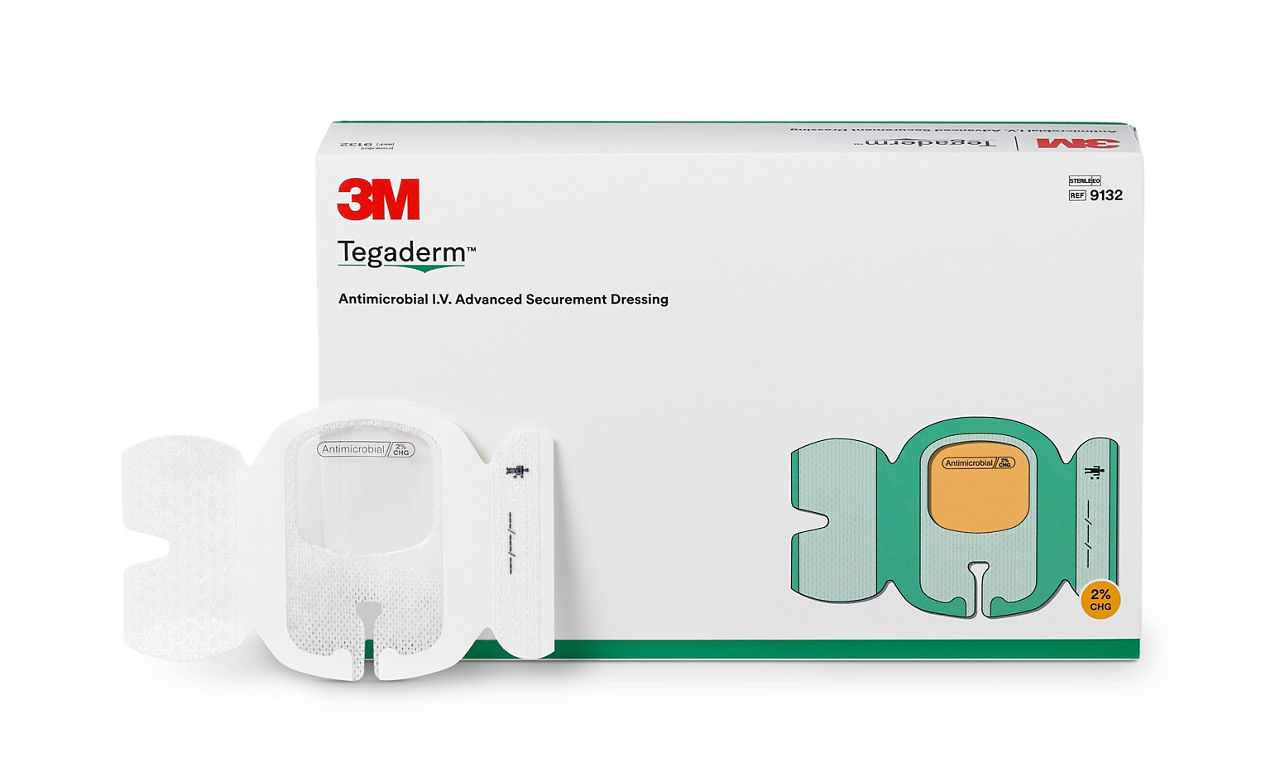
Tegaderm™ Antimicrobial I.V. Advanced Securement Dressing 9132
Product details
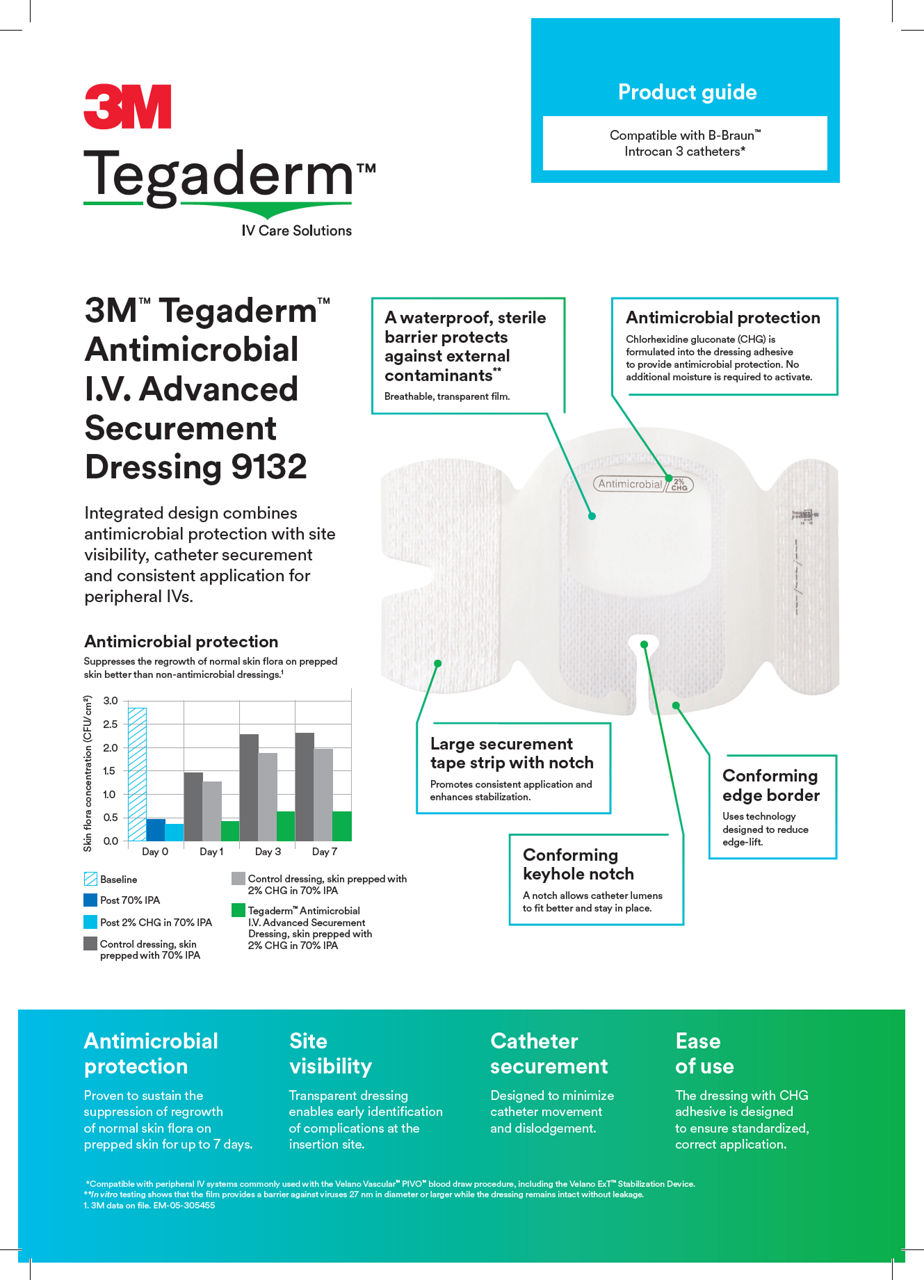
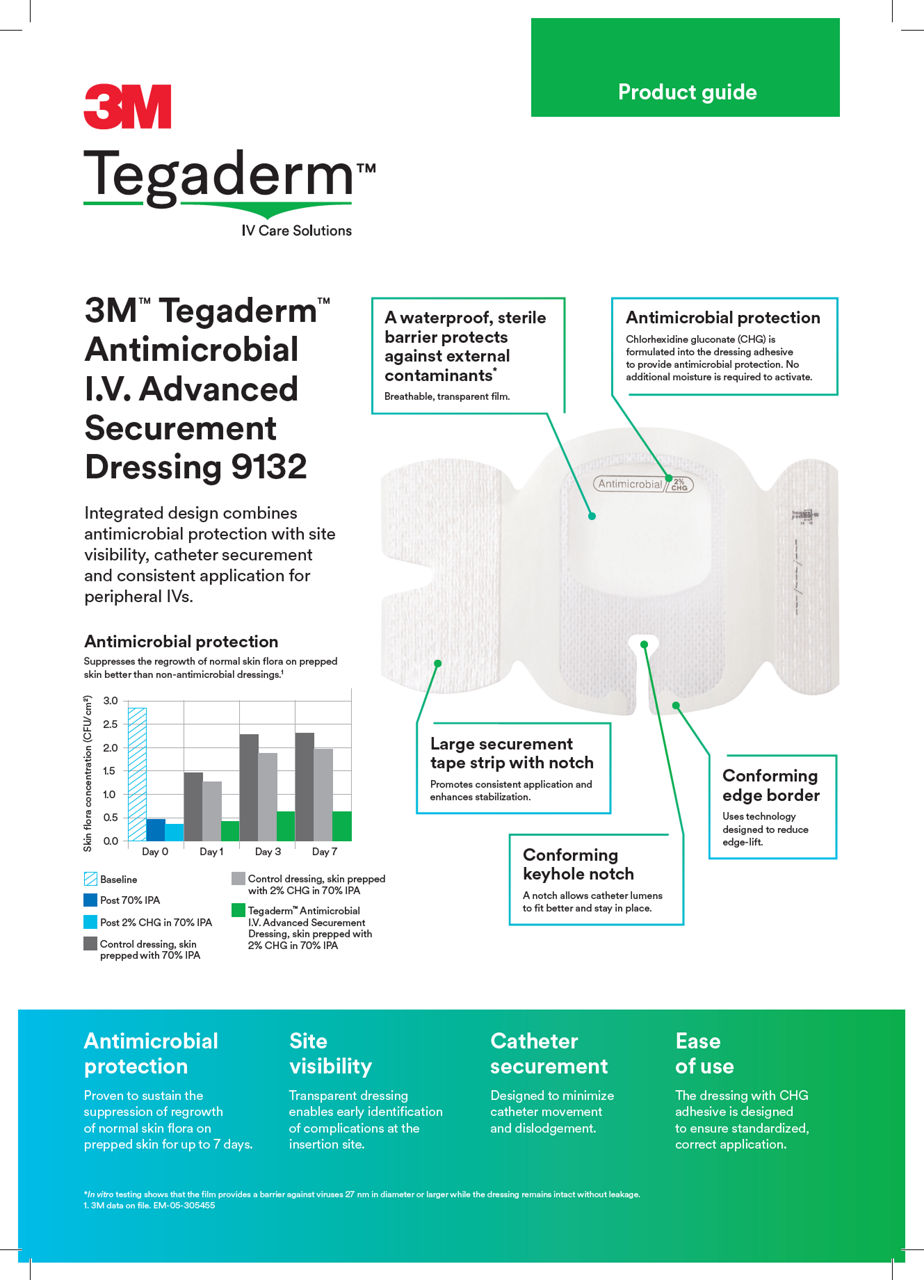
This antimicrobial IV securement dressing has a breathable, transparent film that provides a waterproof, sterile barrier to protect against external contaminants.* The CHG is formulated into the adhesive to provide antimicrobial protection without additional moisture required to activate.
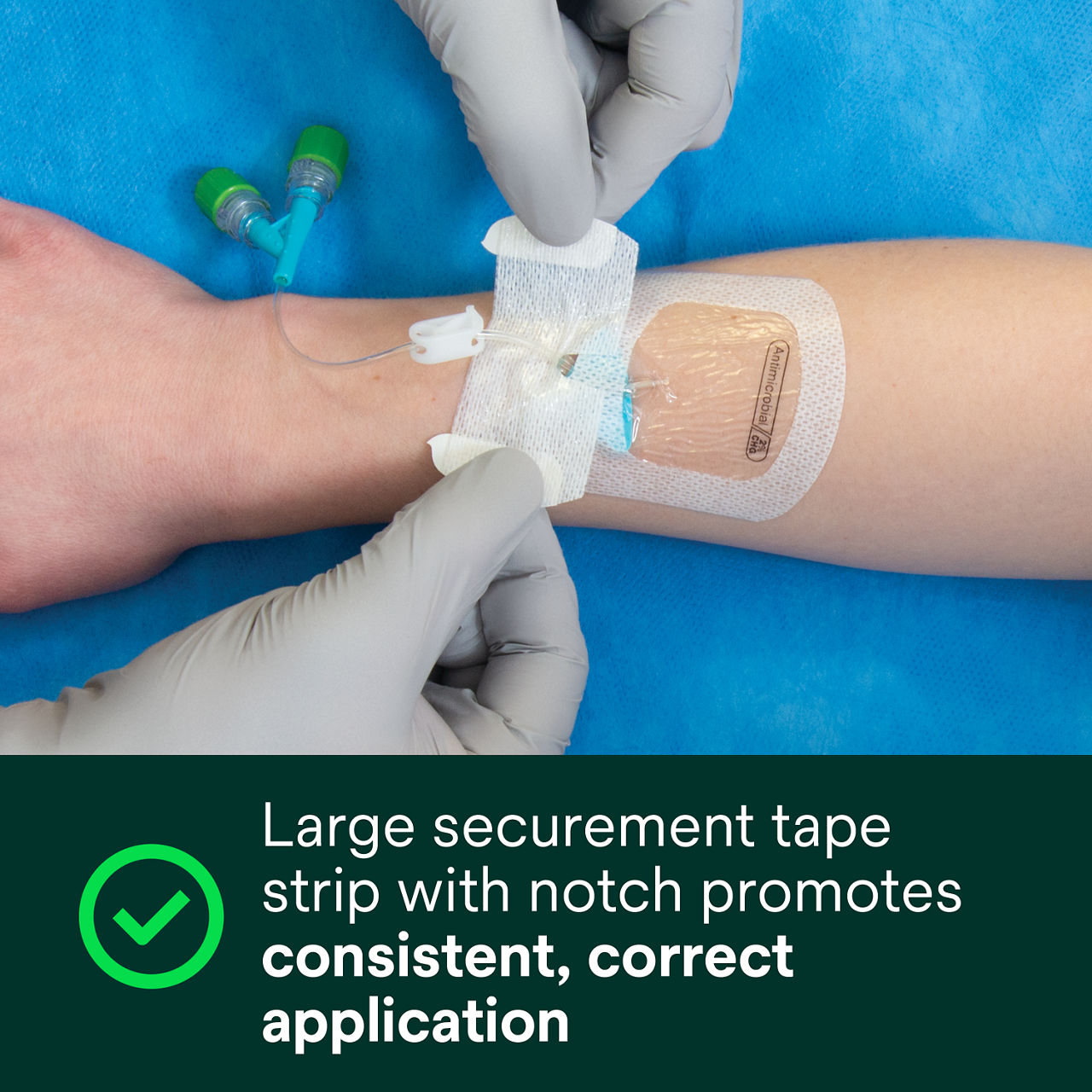
A conforming keyhole notch allows catheter lumens to fit better and stay in place, and the conforming edge border uses technology designed to reduce edge-lift. A securement tape strip with a notch promotes consistent application and enhances stabilization, and a documentation tape strip pre-printed for documenting dressing changes provides additional securement.
Note/Important Safety Warning: Not for use on premature infants or persons with known sensitivity to CHG. This device is not intended to treat, prevent or reduce catheter-related bloodstream infections (CRBSIs) or other percutaneous device-related infections. This device has not been studied in a randomized clinical study to determine its effectiveness in preventing such infections. Reference the Instructions for Use for more information.
*In vitro testing shows that the film provides a barrier against viruses 27 nm in diameter or larger while the dressing remains intact without leakage.
- Antimicrobial IV securement dressing with 2% CHG formulated into the adhesive offers antimicrobial protection without requiring additional moisture to activate
- Integrated design combines antimicrobial protection with site visibility, catheter securement and consistent application
- Proven to suppress regrowth of skin flora for up to 7 days for sustained antimicrobial protection
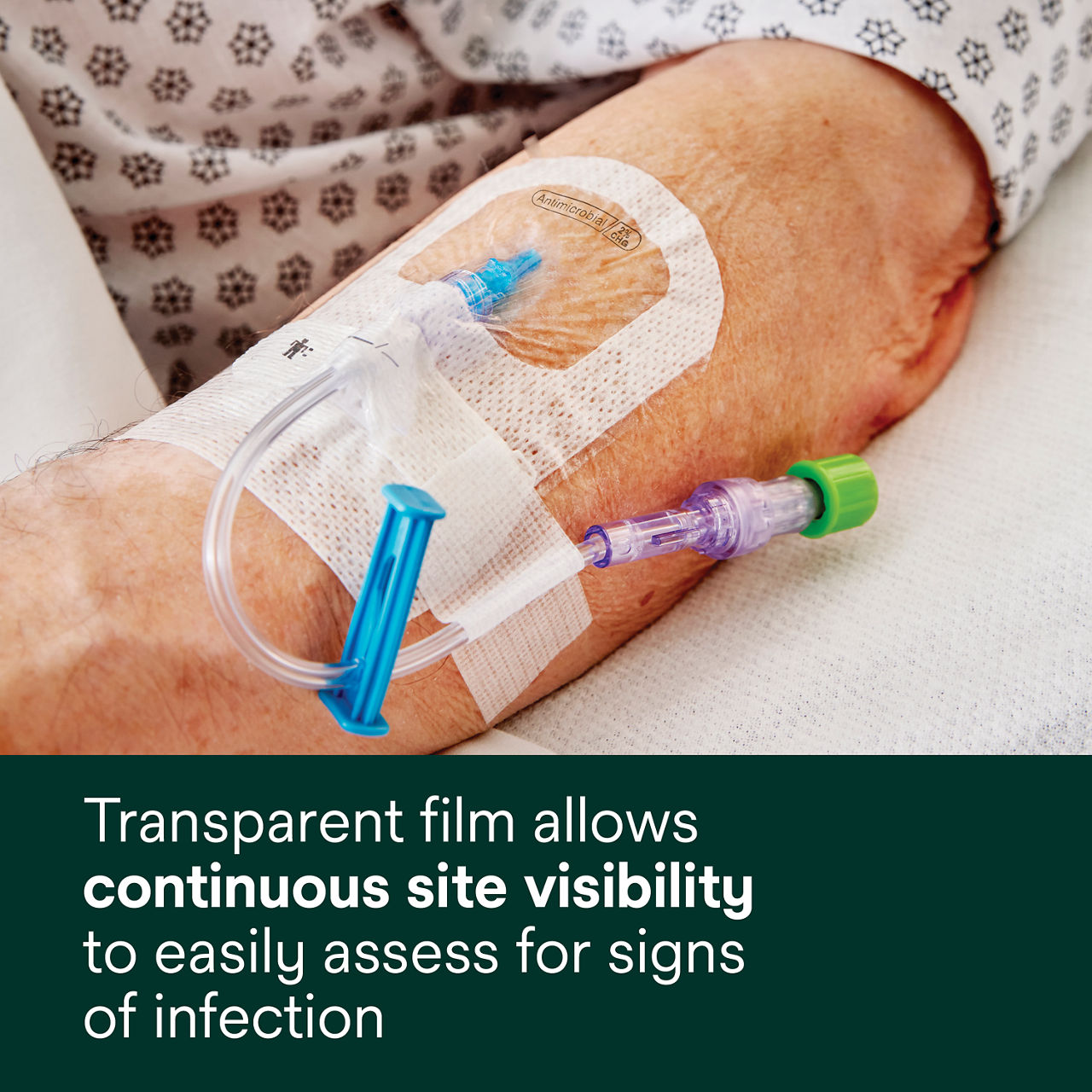
- Transparent film allows continuous site visibility toeasily assess for early signs of infection
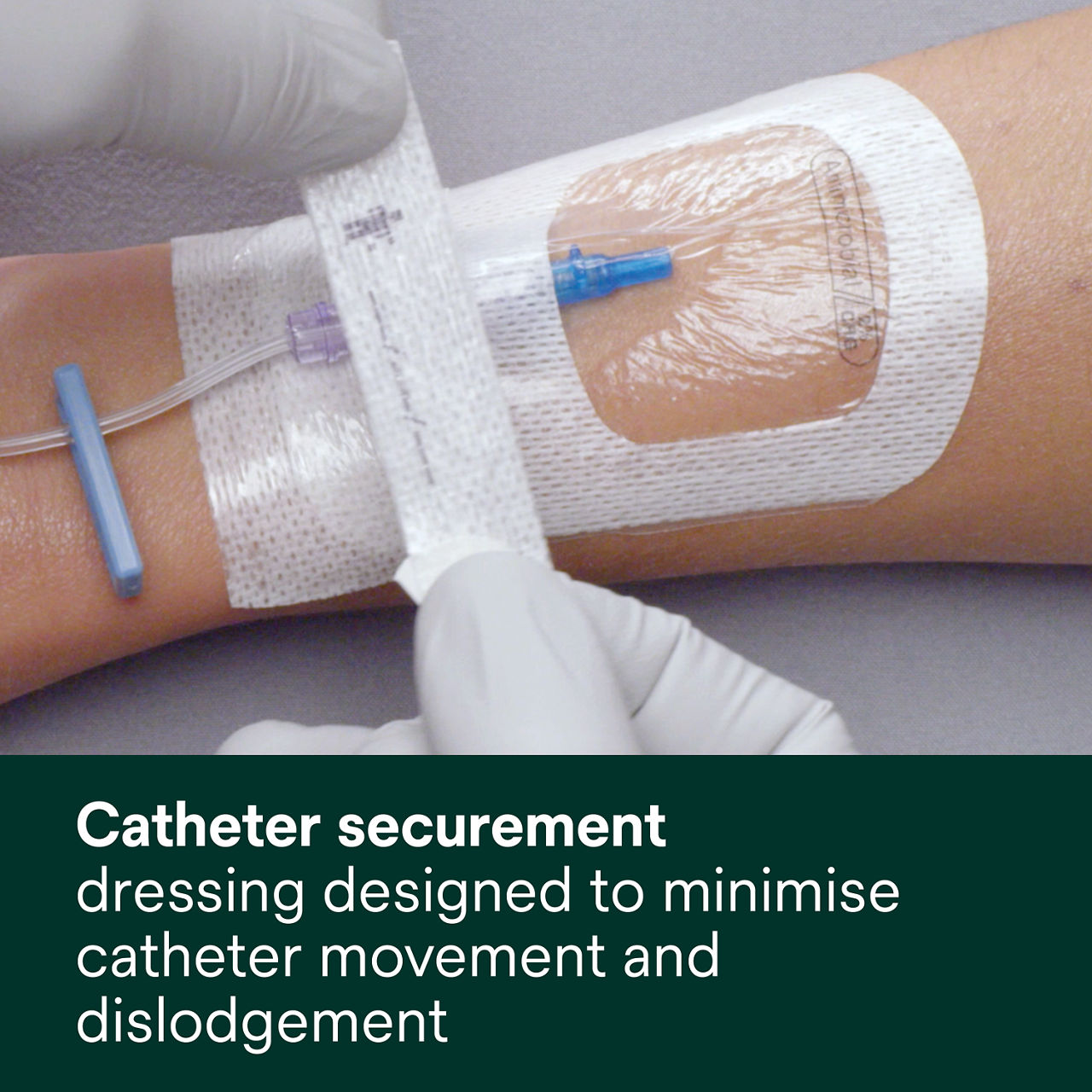
- Designed to minimise catheter movement and dislodgement
- Large securement tape strip with notch promotes consistent, correct application
- Waterproof, sterile barrier protects against external contaminants*
Suggested Applications
- Peripheral IV catheters including Midlines and Mini-Midlines
- The dressing is compatible for use with major skin antiseptics (i.e. IPA, PVPI, and Chlora prep®)
- One-handed application is possible
- Radiologically transparent. The dressing is compatible with MRI (Magnetic Resonance Imaging) (Does not distort the image). MR safe
- The dressing is compatible with the majority of materials for PIV catheters, including polyurethane, silicone, BD Vialon™
- The dressing is compatible for use with No-Sting Barrier Film (note: not closer than within 2 cm of catheter insertion site)
Legal
*In vitro testing shows that the film provides a barrier against viruses 27 nm in diameter or larger while the dressing remains intact without leakage.Product specifications
| Brand |
Brand
Tegaderm™ |
| Overall Width (Metric) |
Overall Width (Metric)
7 cm |
| Overall Length (Metric) |
Overall Length (Metric)
8.5 cm |
| OverallLengthImperial |
OverallLengthImperial
3.35 in |
| Dressing Type |
Dressing Type
Film |
| Category name |
Category name
Antimicrobial IV Dressings |
| Overall Width (Imperial) |
Overall Width (Imperial)
2.76 in |