3M Prevena 治療用於整形外科
通過Prevena治療增強整形手術效果
一項對16項不同整形手術程序的同行評審後的元分析顯示,Prevena治療顯著地降低了各種手術部位併發症(SSCs)的風險,並且與標準護理敷料相比,有助於改善健康經濟結果。1
† 本計算基於本研究報告中相對患者組發生率的推導。統計學上顯著 (p<0.05)。
*相對風險降低
請在https://eifu.solventum.com/查看完整的使用指示和限制。
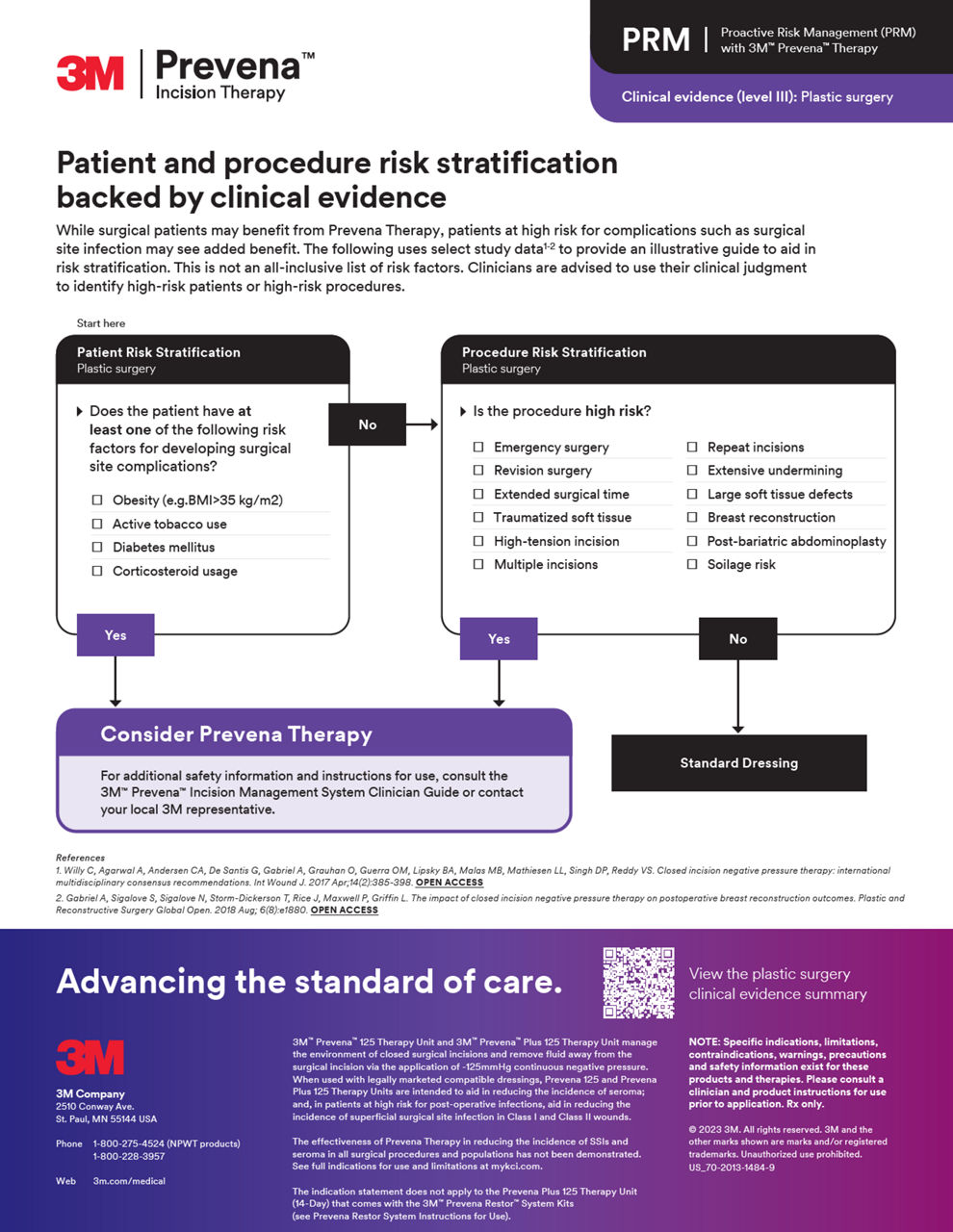
積極風險管理 (PRM)
利用Prevena治療,獲取全面資源以將PRM納入您的實踐,通過證實的術後好處提升您對患者的護理標準。
Prevena 治療視頻資源
案例研究
探索我們精選的案例研究,其中包括受尊敬的外科醫生和專家,展示了Prevena治療在各種外科情境中的效果。
探索更多
摘要
探索與Prevena治療相關主題的著名研究論文中的摘要,提供寶貴的科學發現和觀點。
NOTE:
Specific indications, limitations, contraindications, warnings, precautions and safety information exist for these products and therapies. Please consult a clinician and product instructions for use prior to application. Rx only.
Indication(s) For Use / Intended Use: US FDA Cleared: Only for Use in the United States:
Prevena Dressing used with Prevena Therapy Units: PREVENA™ 125 and PREVENA PLUS™ 125 Therapy Units manage the environment of closed surgical incisions and remove fluid away from the surgical incision via the application of -125mmHg continuous negative pressure. When used with legally marketed compatible PREVENA™ dressings for up to seven days, PREVENA™ 125 and PREVENA PLUS™ 125 Therapy Units are intended to aid in reducing the incidence of seroma; and in patients at high risk for post-operative infections, aid in reducing the incidence of superficial surgical site infection in Class I and Class II wounds.
Limitations:
- The device is not intended to treat surgical site infection or seroma.
- Safety and effectiveness in pediatric population (<22 years old) have not been evaluated.
- Safety and effectiveness in Class III (Contaminated) and Class IV (Dirty/Infected) wounds have not been demonstrated. Furthermore, Class IV surgical wounds are not expected to be closed primarily, and the subject device should only be used on closed surgical incisions.
- The device has not been demonstrated to reduce deep incisional and organ space surgical site infections.
- The device has not been demonstrated to be effective in reducing the incidence of surgical site infection and seroma in all surgical procedures and patient populations; therefore, the device may not be recommended for routine use to reduce the incidence of surgical site infection and seroma.
- Please refer to the ‘Summary of Clinical Information’ section for the specific surgical procedures and patient populations included in the clinical studies. Surgeons should continue to follow the ‘Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection’2 and the ‘American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines’3 for best practices in preventing surgical site infection. US FDA Cleared: Dressings/ Systems (Prevena Dressings used with compatible Solventum NPWT units - ActiVAC, Ulta, and RX4) and applicable OUS countries that leverage US Indication: The PREVENA™, PREVENA PLUS™, PREVENA DUO™, and PREVENA RESTOR™ Incision Management Systems are intended to manage the environment of surgical incisions that continue to drain following sutured or stapled closure by maintaining a closed environment and removing exudates via the application of negative pressure wound therapy.
參考文獻:
- Gabriel A, Singh D, Silverman RP, Collinsworth A, Bongards C, Griffin L. ePlasty. 2023 Mar 31;23:e22. Closed incision negative pressure therapy versus standard of care over closed plastic surgery incisions in the reduction of surgical site complications: A systematic review and meta-analysis of comparative studies
- Berríos-Torres, S. I., Umscheid, C. A., Bratzler, D. W., Leas, B., Stone, E. C., Kelz, R. R., Reinke, C. E., Morgan, S., Solomkin, J. S., Mazuski, J. E., Dellinger, E. P., Itani, K. M., Berbari, E. F., Segreti, J., Parvizi, J., Blanchard, J., Allen, G., Kluytmans, J. A., Donlan, R., & Schecter, W. P. (2017). Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surgery, 152(8), 784. https://doi.org/10.1001/jamasurg.2017.0904opens in a new tab
- Ban, K. A., Minei, J. P., Laronga, C., Harbrecht, B. G., Jensen, E. H., Fry, D. E., Itani, K. M. F., Dellinger, P. E., Ko, C. Y., & Duane, T. M. (2017). American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 update. Journal of the American College of Surgeons, 224(1), 59–74. https://doi.org/10.1016/j.jamcollsurg.2016.10.029opens in a new tab