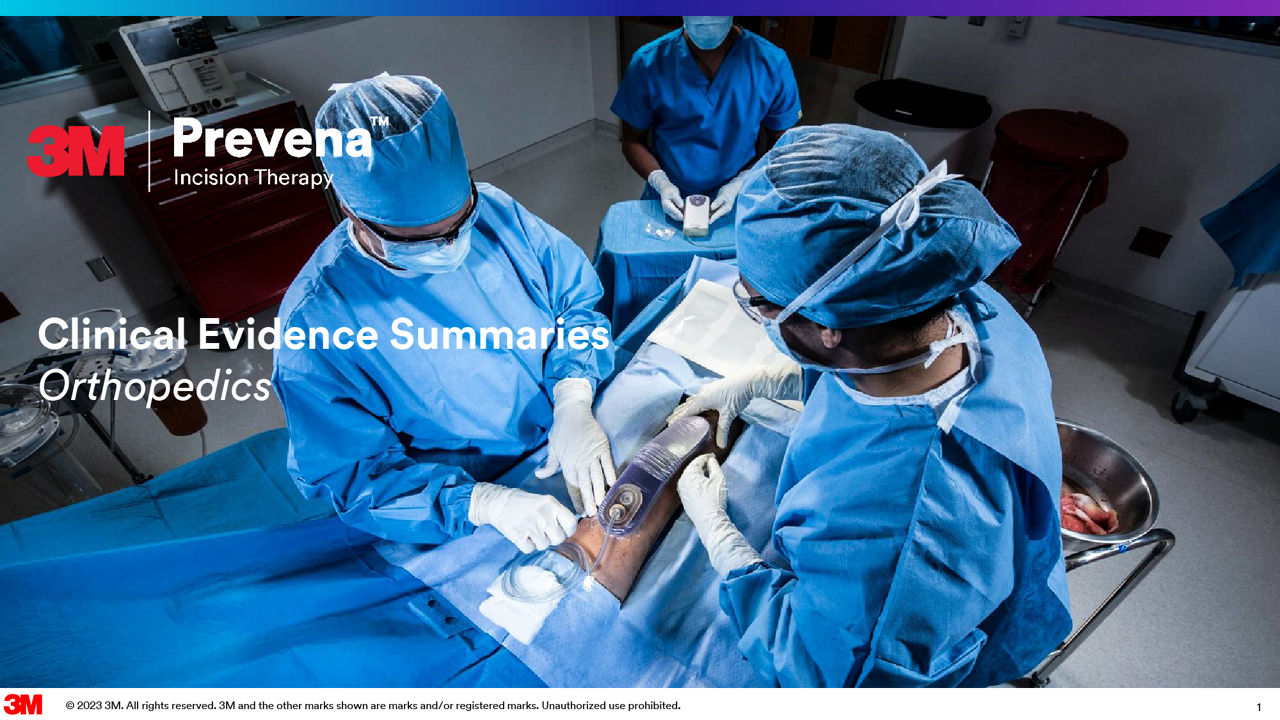
3M Prevena 治療用於骨科手術
使用Prevena治療提升骨科手術效果
一項對於骨科關節置換手術的12項研究進行的同行評審後的整合分析顯示,Prevena治療顯著降低了各種手術部位併發症(SSCs)的風險,同時與標準護理敷料相比,有助於改善健康經濟結果。¹
SSCs¹,* 減少
8項研究;p<0.001†
外科手術部位感染(SSIs)的減少¹,*
7項研究;p=0.016†
減少血清瘤¹,*
3項研究;p=0.008†
*基於此研究報告的風險比率計算的相對風險降低。
†計算基於此研究報告的相對病人群發生率。統計顯著性 (p<0.05)。
請參閱完整的使用指示和限制,網址位於 https://eifu.solventum.com。
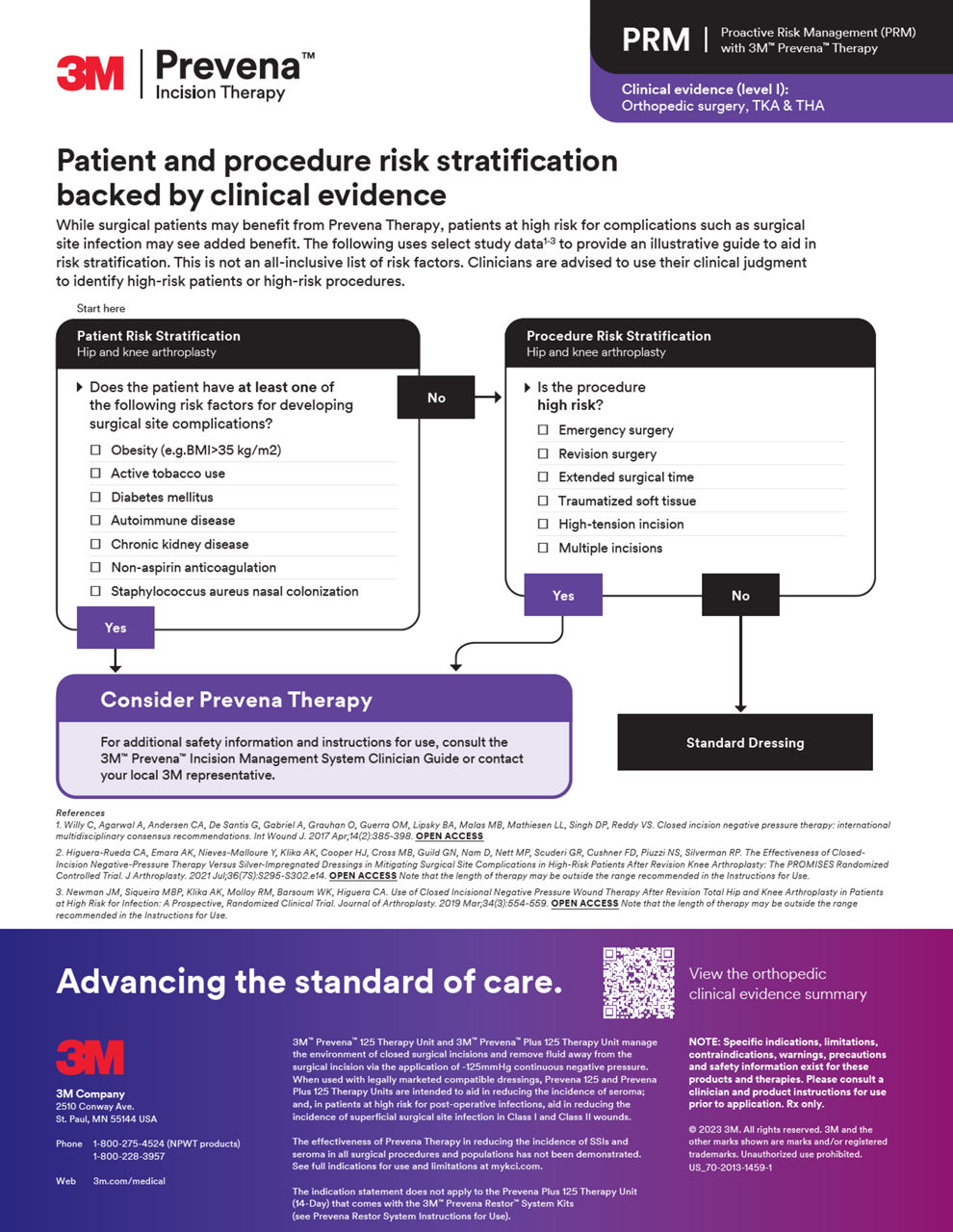
積極風險管理 (PRM)
利用Prevena治療,進入全面資源以將PRM整合到您的實踐中,有助於通過證明的術後效益提升您的病患護理標準。
Prevena 治疗视频资源
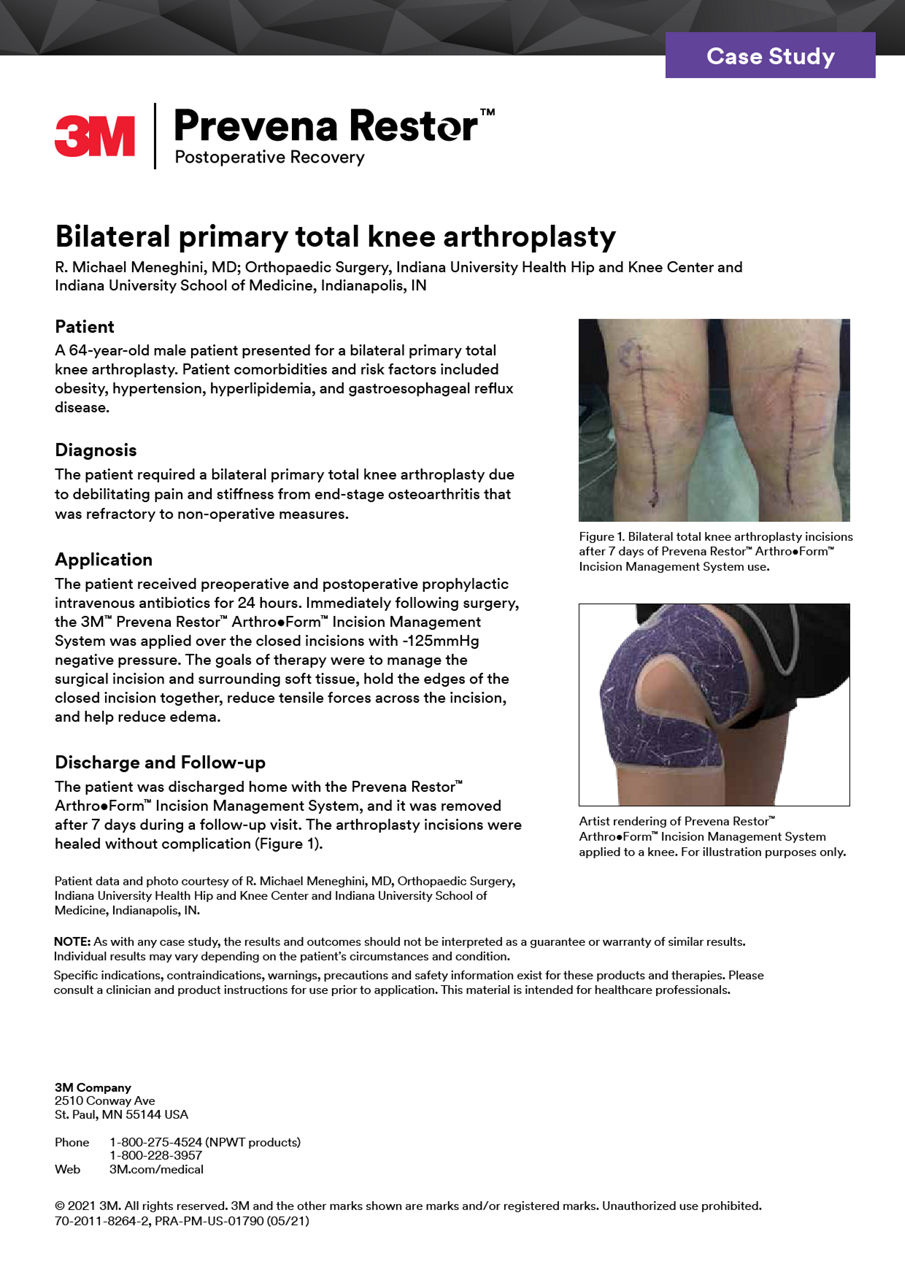
案例研究
探索我們精選的案例研究,其中包括享譽盛名的外科醫生和專家,展示了Prevena治療在各種手術情景中的有效性。
探索更多
摘要
探索與Prevena治療相關主題的著名研究論文中的摘要,提供有價值的科學發現和觀點。
- 閉合切口負壓治療與含銀敷料在減少修復性膝關節置換術後高風險患者手術部位併發症的有效性:PROMISES 隨機對照試驗
- 使用負壓傷口治療預防血清腫和治療全髖關節置換術後的手術切口
- 閉合切口負壓治療與標準護理在減少膝和髖關節置換手術切口的手術部位併發症:比較研究的系統性評價和元分析
- 在高感染風險患者修復性全髖和膝關節置換術後使用閉合切口負壓傷口治療:前瞻性隨機臨床試驗
- 在高風險全膝關節置換患者中,手術部位併發症的負壓傷口治療與含銀敷料的比較:配對隊列研究
- 修復性全膝關節置換術後使用閉合切口負壓治療的成本效益:隨機臨床試驗的次級分析
- 修復性髖和膝手術後閉合切口負壓治療與抗菌敷料的比較研究
- 在原發性髖和膝關節置換術中使用風險分層算法以減少表層手術部位併發症
- 閉合切口負壓治療對全髖和膝關節置換術後感染和手術部位併發症的影響
NOTE:
Specific indications, limitations, contraindications, warnings, precautions and safety information exist for these products and therapies. Please consult a clinician and product instructions for use prior to application. Rx only.
Indication(s) For Use / Intended Use: US FDA Cleared: Only for Use in the United States:
Prevena Dressing used with Prevena Therapy Units: PREVENA™ 125 and PREVENA PLUS™ 125 Therapy Units manage the environment of closed surgical incisions and remove fluid away from the surgical incision via the application of -125mmHg continuous negative pressure. When used with legally marketed compatible PREVENA™ dressings for up to seven days, PREVENA™ 125 and PREVENA PLUS™ 125 Therapy Units are intended to aid in reducing the incidence of seroma; and in patients at high risk for post-operative infections, aid in reducing the incidence of superficial surgical site infection in Class I and Class II wounds.
Limitations:
- The device is not intended to treat surgical site infection or seroma.
- Safety and effectiveness in pediatric population (<22 years old) have not been evaluated.
- Safety and effectiveness in Class III (Contaminated) and Class IV (Dirty/Infected) wounds have not been demonstrated. Furthermore, Class IV surgical wounds are not expected to be closed primarily, and the subject device should only be used on closed surgical incisions.
- The device has not been demonstrated to reduce deep incisional and organ space surgical site infections.
- The device has not been demonstrated to be effective in reducing the incidence of surgical site infection and seroma in all surgical procedures and patient populations; therefore, the device may not be recommended for routine use to reduce the incidence of surgical site infection and seroma.
- Please refer to the ‘Summary of Clinical Information’ section for the specific surgical procedures and patient populations included in the clinical studies. Surgeons should continue to follow the ‘Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection’2 and the ‘American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines’3 for best practices in preventing surgical site infection. US FDA Cleared: Dressings/ Systems (Prevena Dressings used with compatible Solventum NPWT units - ActiVAC, Ulta, and RX4) and applicable OUS countries that leverage US Indication: The PREVENA™, PREVENA PLUS™, PREVENA DUO™, and PREVENA RESTOR™ Incision Management Systems are intended to manage the environment of surgical incisions that continue to drain following sutured or stapled closure by maintaining a closed environment and removing exudates via the application of negative pressure wound therapy.
參考文獻:
- Cooper HJ, Silverman RP, Collinsworth A, Bongards C, Griffin L. Closed Incision Negative Pressure Therapy vs Standard of Care Over Closed Knee and Hip Arthroplasty Surgical Incisions in the Reduction of Surgical Site Complications: A Systematic Review and Meta-analysis of Comparative Studies. Arthroplasty Today. 2023;21:101120. Published 2023 Apr 3. doi:10.1016/j.artd.2023.101120
- Berríos-Torres, S. I., Umscheid, C. A., Bratzler, D. W., Leas, B., Stone, E. C., Kelz, R. R., Reinke, C. E., Morgan, S., Solomkin, J. S., Mazuski, J. E., Dellinger, E. P., Itani, K. M., Berbari, E. F., Segreti, J., Parvizi, J., Blanchard, J., Allen, G., Kluytmans, J. A., Donlan, R., & Schecter, W. P. (2017). Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surgery, 152(8), 784. https://doi.org/10.1001/jamasurg.2017.0904
- Ban, K. A., Minei, J. P., Laronga, C., Harbrecht, B. G., Jensen, E. H., Fry, D. E., Itani, K. M. F., Dellinger, P. E., Ko, C. Y., & Duane, T. M. (2017). American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 update. Journal of the American College of Surgeons, 224(1), 59–74. https://doi.org/10.1016/j.jamcollsurg.2016.10.029