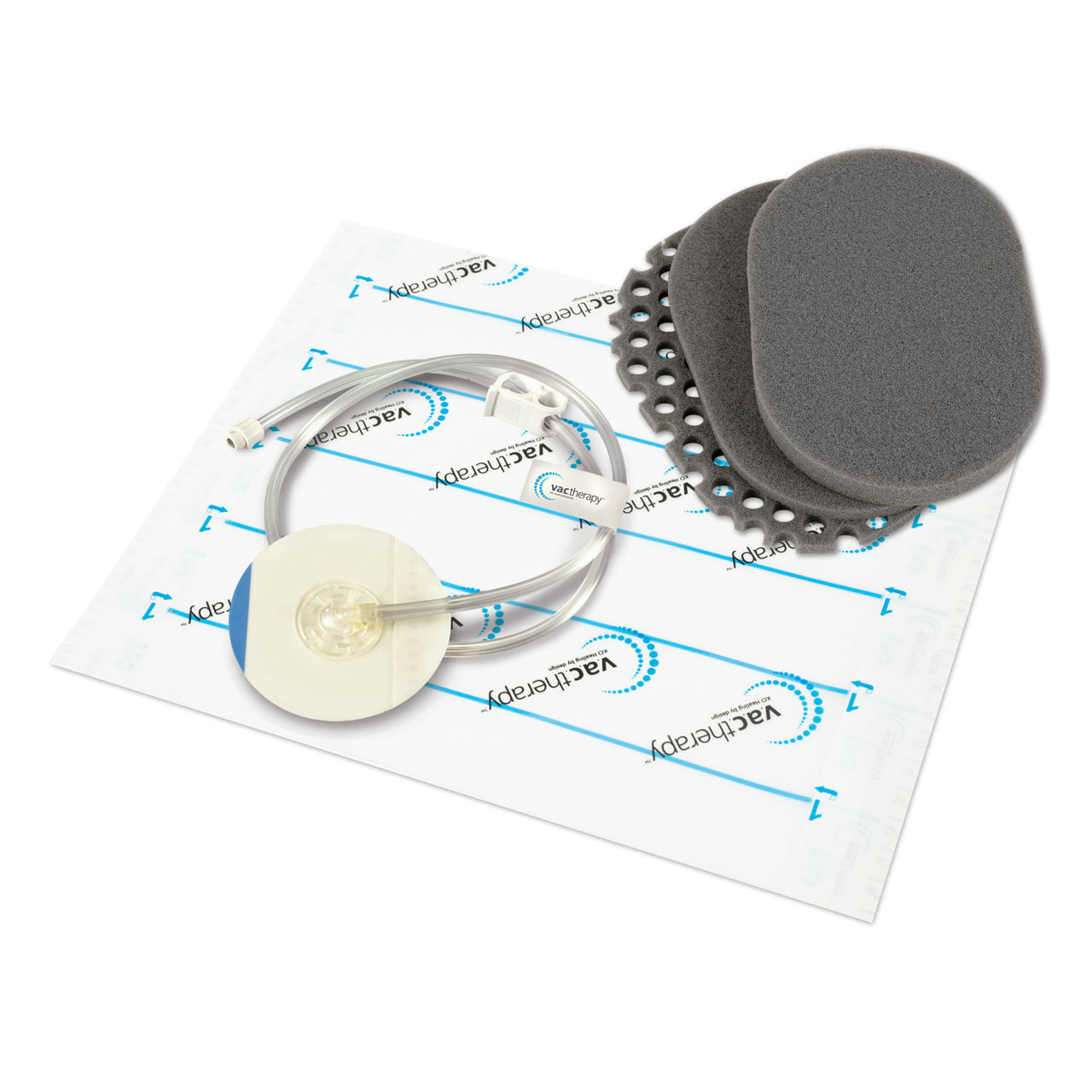
V.A.C. Veraflo Cleanse Choice™ Dressing
Product details
Designed specifically for use with Veraflo Therapy, provides a wound cleansing option for clinicians when surgical debridement must be delayed or is not possible or appropriate.¹ V.A.C. Veraflo Cleanse Choice™ Dressing provides “mechanical” movement at the wound surface in combination with cyclic delivery and dwell of topical solutions to help disrupt, soften, solubilize and remove thick exudate (such as or e.g. slough) and non-viable tissue.¹ V.A.C. Veraflo Cleanse Choice Dressing with Veraflo Therapy is ideal for dirty wounds needing immediate wound cleansing.¹ 1 Téot L, Boissiere F, Fluieraru S. Novel foam dressing using negative pressure wound therapy with instillation to remove thick exudate. Int Wound J. 2017 Oct;14(5):842-848. doi: 10.1111/iwj.12719. Epub 2017 Feb 28. PMID: 28244217.
- Three foam layers to provide application options for wounds with varying depths
- "Honeycomb" pattern contact layer
- Conformable thin cover layer for shallower wounds and thick cover layer for deeper wounds
- Dressing available in Medium & Large sizes to minimize waste Medium Size : 18.0cm x 12.5cm x Varying Thicknesses (0.8cm – 1.6cm), dressing kit includes: 4x sheets V.A.C.® Advanced Drape, 1x V.A.C. VeraT.R.A.C.™ Pad, 4x Cavilon No Sting Barrier Film, 1x disposable ruler/foam quantity label Large Size: 25.6cm x 15.0cm x VaryingThicknesses (0.8cm – 1.6cm), dressing kit includes: 6x sheets V.A.C.® Advanced Drape, 1x V.A.C. VeraT.R.A.C. Duo™ Pad, 5x Cavilon No Sting Barrier Film, 1x disposable ruler/foam quantity label
Legal
¹ Outside US (OUS) Prevena Therapy instructions for use / OUS indication statement ² Wilkes RP, Kilpad DV, Zhao Y, Kazala R, McNulty A. Closed Incision management with negative pressure wound therapy (CIM): biomechanics. Surg Innov. 2012;19(1):67–75. doi:10.1177/1553350611414920. ³ Glaser DA, Farnsworth CL, Varley ES, et al. Negative pressure therapy for closed spine incisions: a pilot study. Wounds. 2012;24(11):308–316. ⁴ Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM): hematoma/seroma and involvement of the lymphatic system. Wound Repair Regen. 2011;19(5):588–596. doi:10.1111/j.1524-475X.2011.00714.x. ⁵ Ferrando PM, Ala A, Bussone R, Bergamasco L, Actis Perinetti F, Malan F. Closed incision negative pressure therapy in oncological breast surgery: comparison with standard care dressings. Plast Reconstr Surg Glob Open. 2018 Jun 15;6(6):e1732. doi:10.1097/GOX000000000000173 ³ Pleger SP, Nink N, Elzien M, Kunold A, Koshty A, Boning A. Reduction of groin wound complications in vascular surgery patients using closed incision negative pressure therapy (ciNPT): a prospective, randomised, single-institution study. Int Wound J. 2018;15(1):75-83. ⁴ Cooper HJ, Bas MA. Closed-incision negative-pressure therapy versus antimicrobial dressings after revision hip and knee surgery: a comparative study. J Arthroplasty. 2016 May;31(5):1047-1052. doi:10.1016/j.arth.2015.11.010 ⁵ Redfern RE, Cameron-Ruetz C, O’Drobinak SK, Chen JT, Beer KJ. Closed incision negative pressure therapy effects on postoperative infection and surgical site complication after total hip and knee arthroplasty. J Arthroplasty. 2017;32:3333-3339. doi:10.1016/j.arth.2017.06.019 ⁶ Newman JM, Siqueira MBP, Klika AK, Molloy RM, Barsoum WK, Higuera CA. Use of closed incisional negative pressure wound therapy after revision total hip and knee arthroplastyProduct specifications
| Size |
Size
Medium, Large |
| Category name |
Category name
NPWT Dressings |
| Trademark2 |
Trademark2
Veraflo™ |
| categoryName |
categoryName
NPWT Dressings |