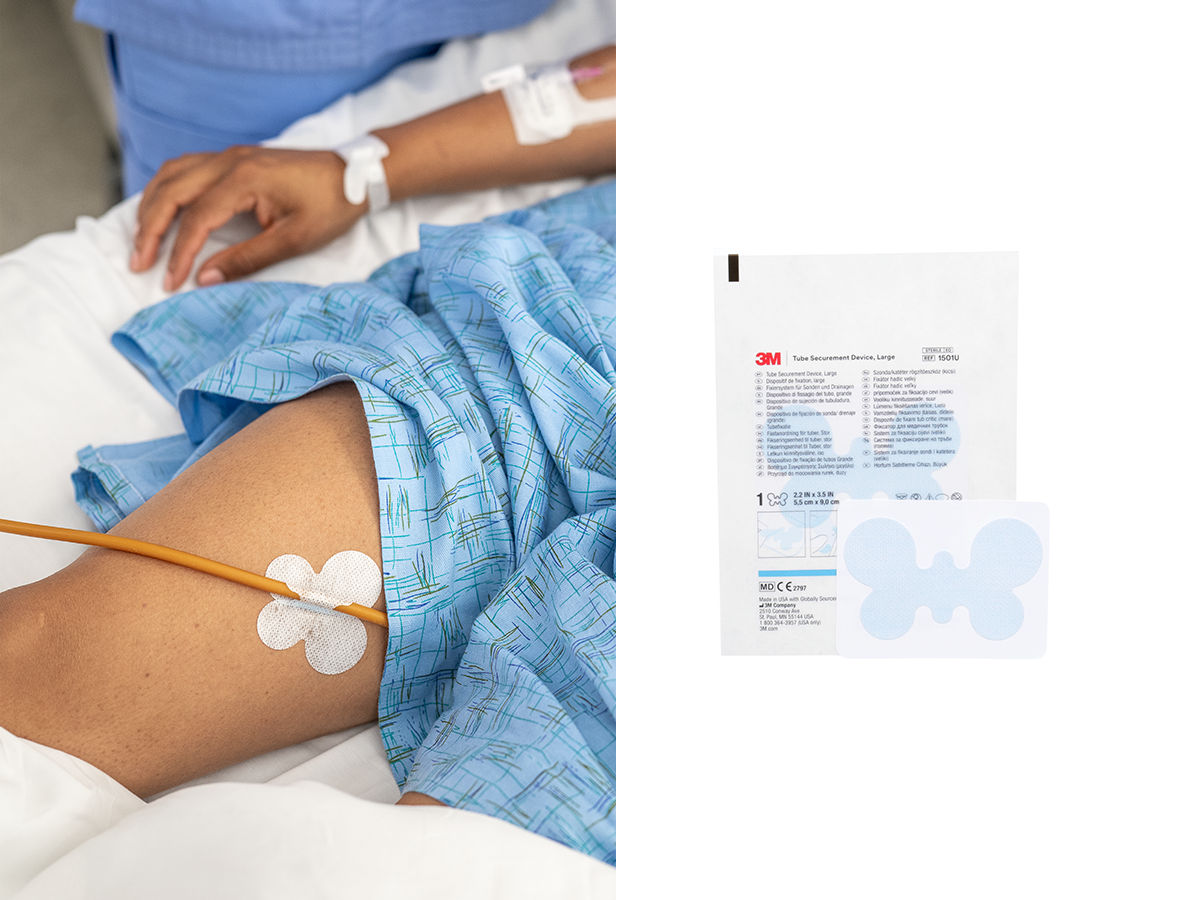
3M™ Tube Securement Device
About the product
The 3M™ Tube Securement Device delivers strong securement and skin performance in an easy-to-use solution. Designed to support medical adhesive-related skin injury (MARSI) and pressure injury prevention practices, it is gentle to skin and is indicated for non-critical tube securement or in conjunction with sutures, primary securement dressings or urinary catheter balloons per facility protocol.
Product details
When it comes to tube securement selection, there are a variety of factors to keep in mind, including securement performance, skin protection and clinician ease of use.
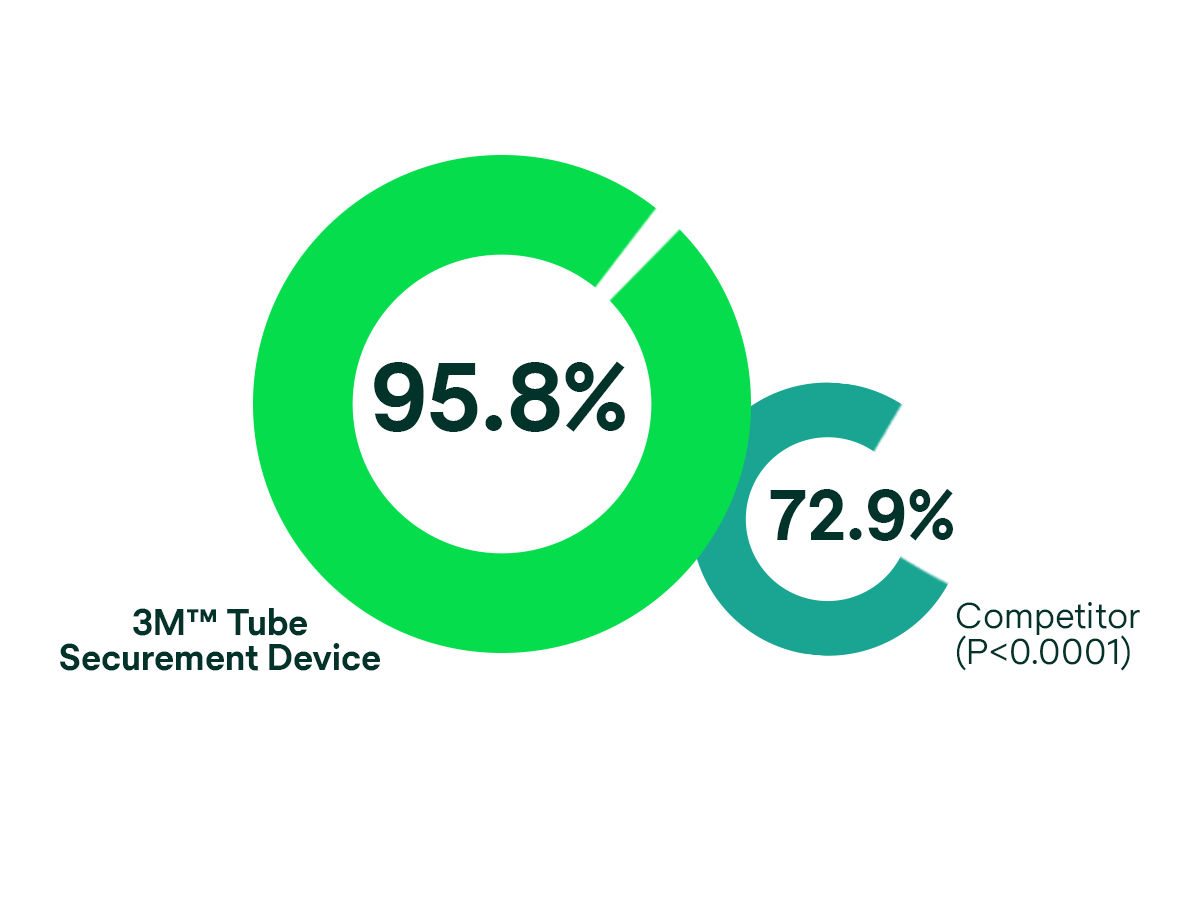
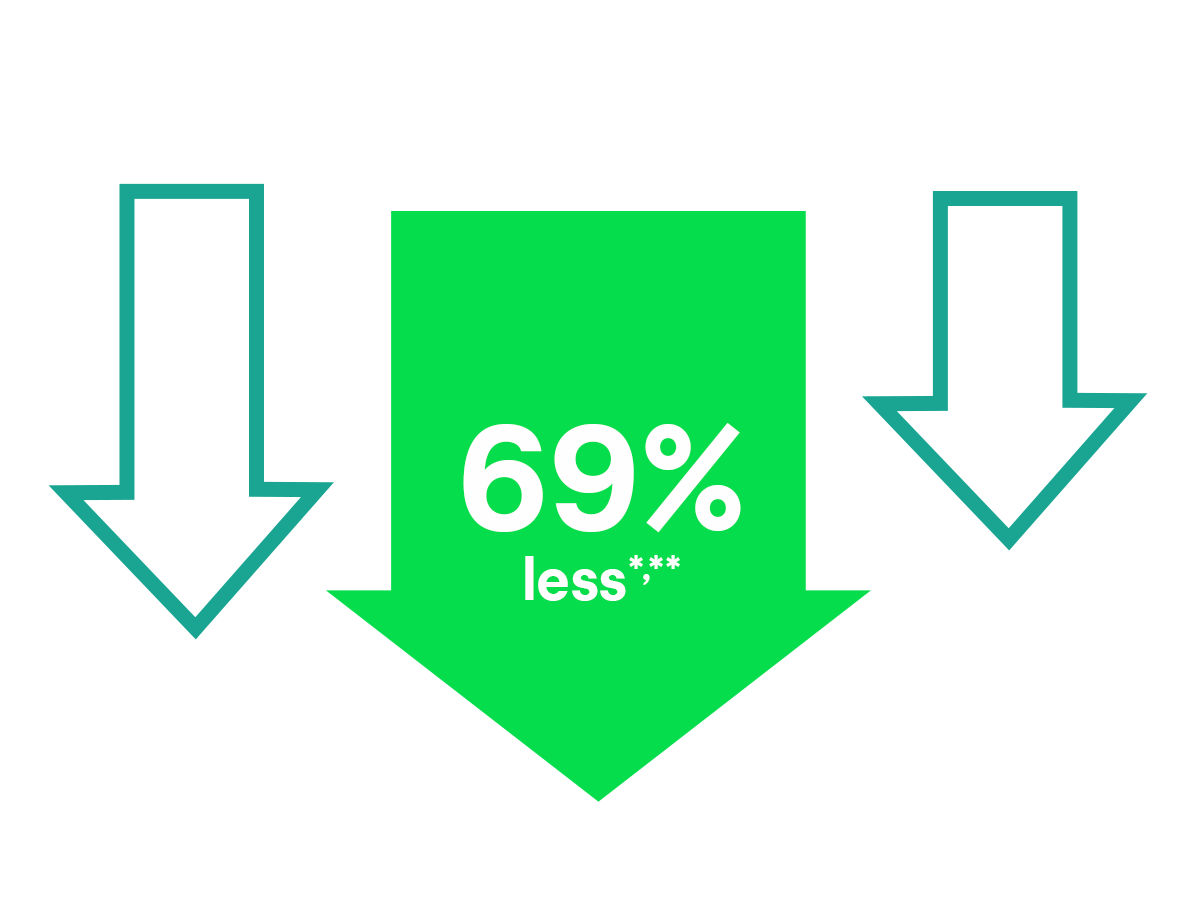
Consider the results of this comparative study on urinary catheter securement: A comparison of the 3M™ Tube Securement Device to the Competitor Stabilisation Device.2
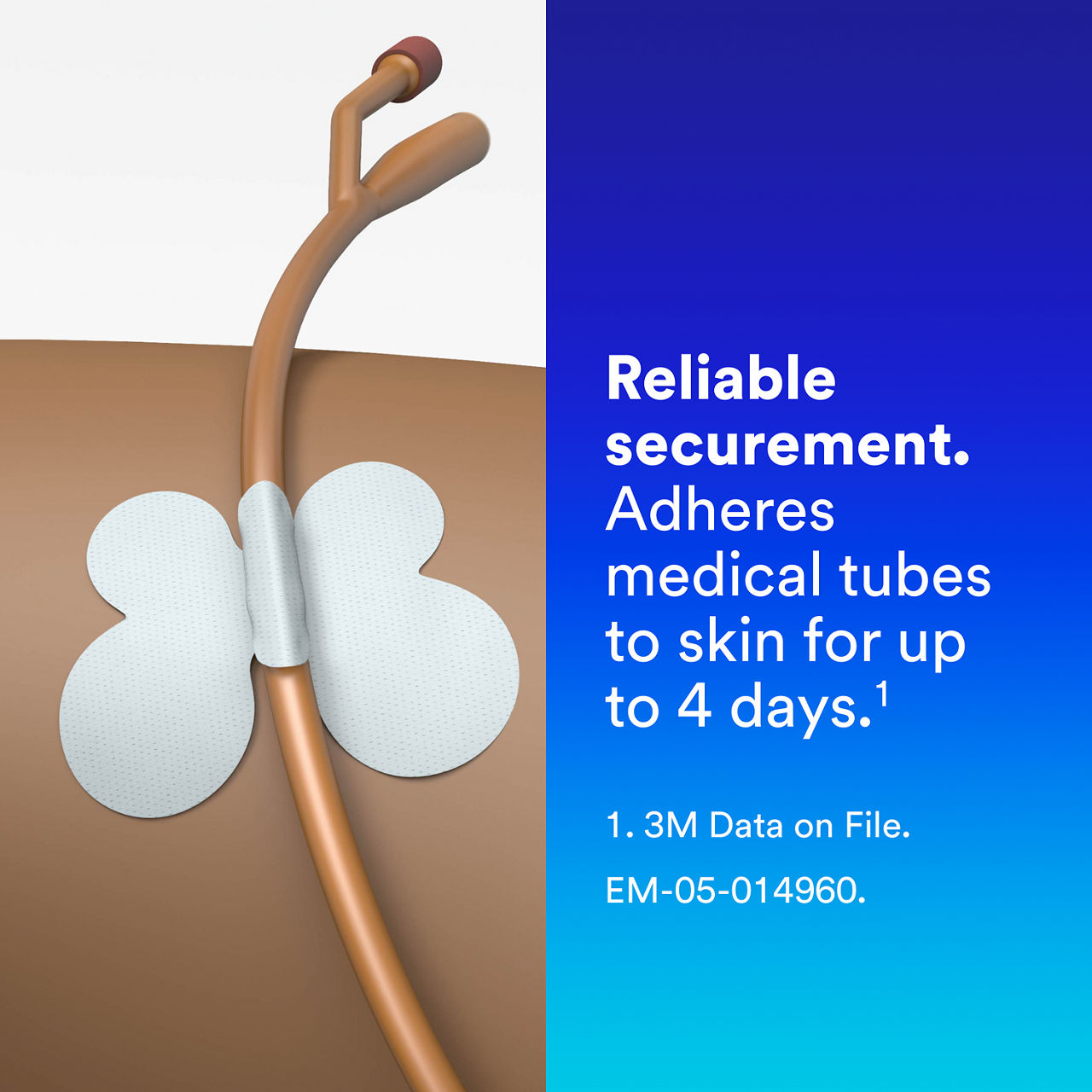
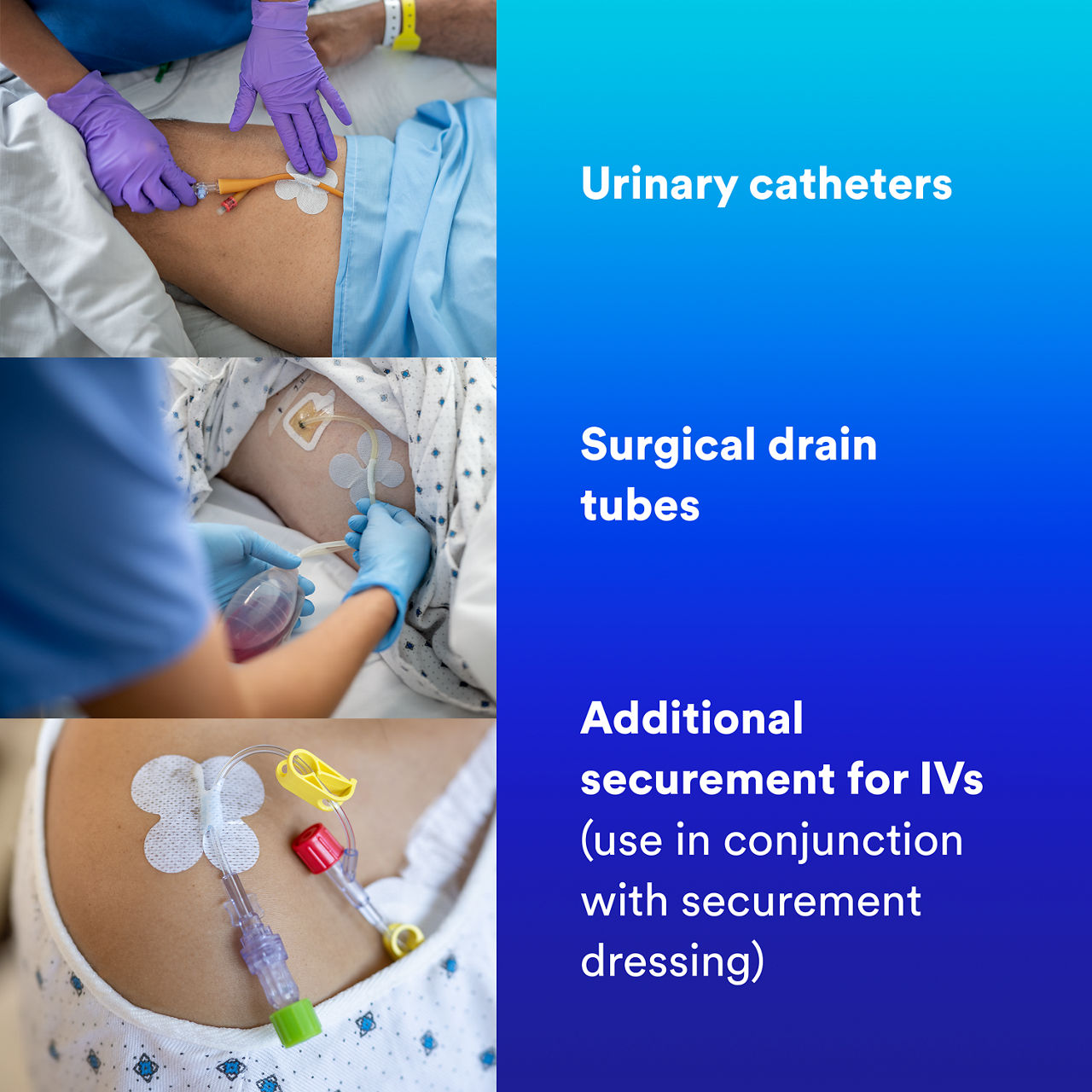
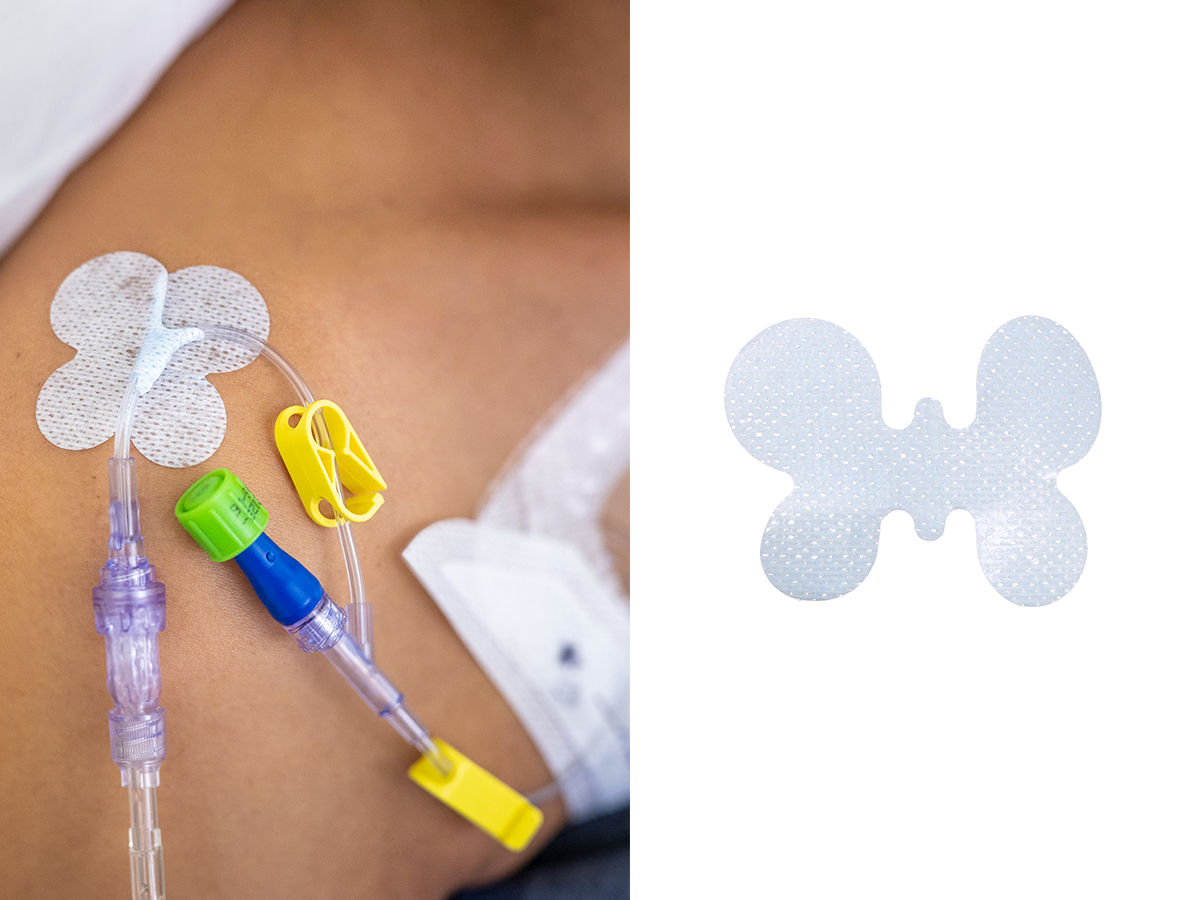
- Reliable securement: Adhesive delivers strong securement, adhering medical tubes such as urinary catheters and surgical drain tubes to skin for up to 4 days¹
- Skin performance: Designed to help reduce the risk of pressure injury with no hard plastic components and a shape to minimise contact between skin and tube
- Skin performance: Designed to deliver the securement power you need while minimising damage to skin upon removal; removes cleanly from skin without removal agent
- Easy-to-use: Easy to apply and remove; allows for standardisation of technique
- Sterile device in single-use packaging
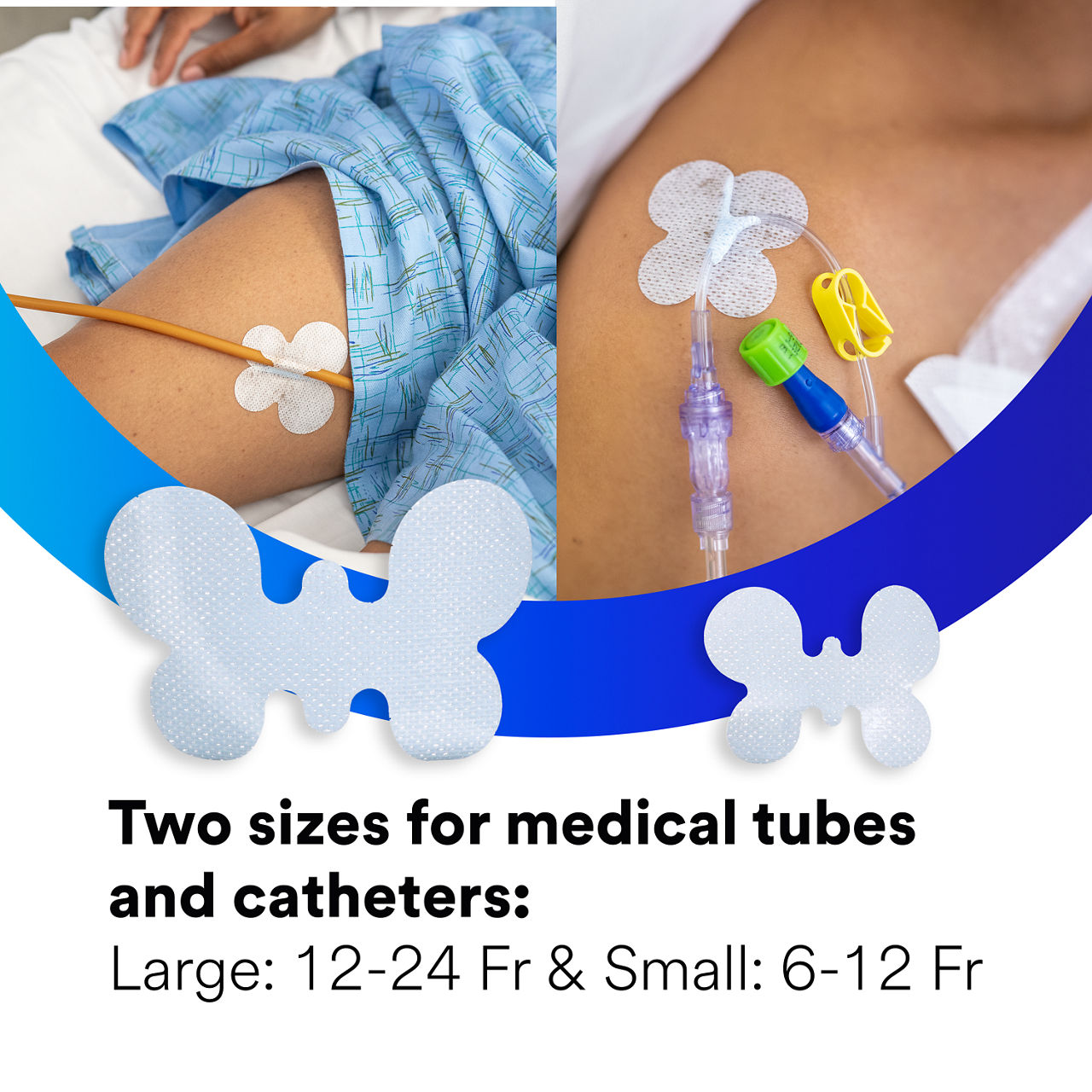
- Secures a variety of non-critical medical tubes and catheters made from silicone, PVC and silicone coated latex to skin, spanning 6-24 French
Suggested Applications
- Indicated for non-critical tube securement or in conjunction with sutures, primary securement dressings and/or urinary catheter balloons per facility protocol.
Legal
¹ 3M Data on File. EM -05 -014960. ¹ ² 3M Data on File. EM-05-014985.Product specifications
| Overall Length (Imperial) |
Overall Length (Imperial)
2.17 in, 1.69 in |
| Overall Width (Metric) |
Overall Width (Metric)
9 cm, 6.1 cm |
| Overall Length (Metric) |
Overall Length (Metric)
5.5 cm, 4.3 cm |
| Size |
Size
Large, Small |
| Category name |
Category name
Securement Dressings & Devices |
| Overall Width (Imperial) |
Overall Width (Imperial)
3.54 in, 2.4 in |