Cavilon™ Advanced Skin Protectant
Product details
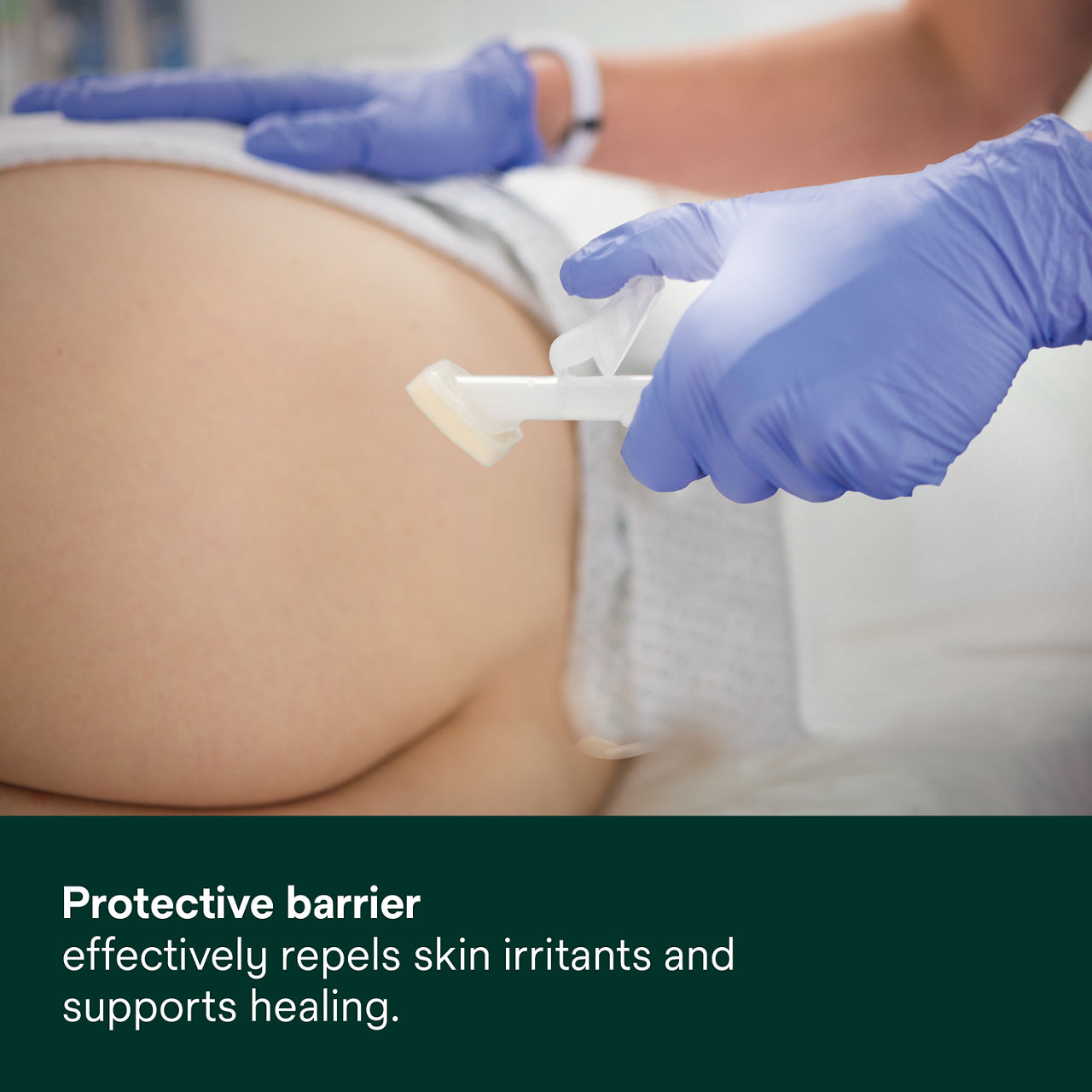
- Provides an effective barrier, which has been shown to reduce the pain of managing incontinence-associated dermatitis (IAD)* and helps to prevent skin damage from body fluids
*Cavilon Advanced skin protectant is not an analgesic
- Lasts up to seven days and may be applied one to three times per week
- Adheres to damag, wet and weepy skin.
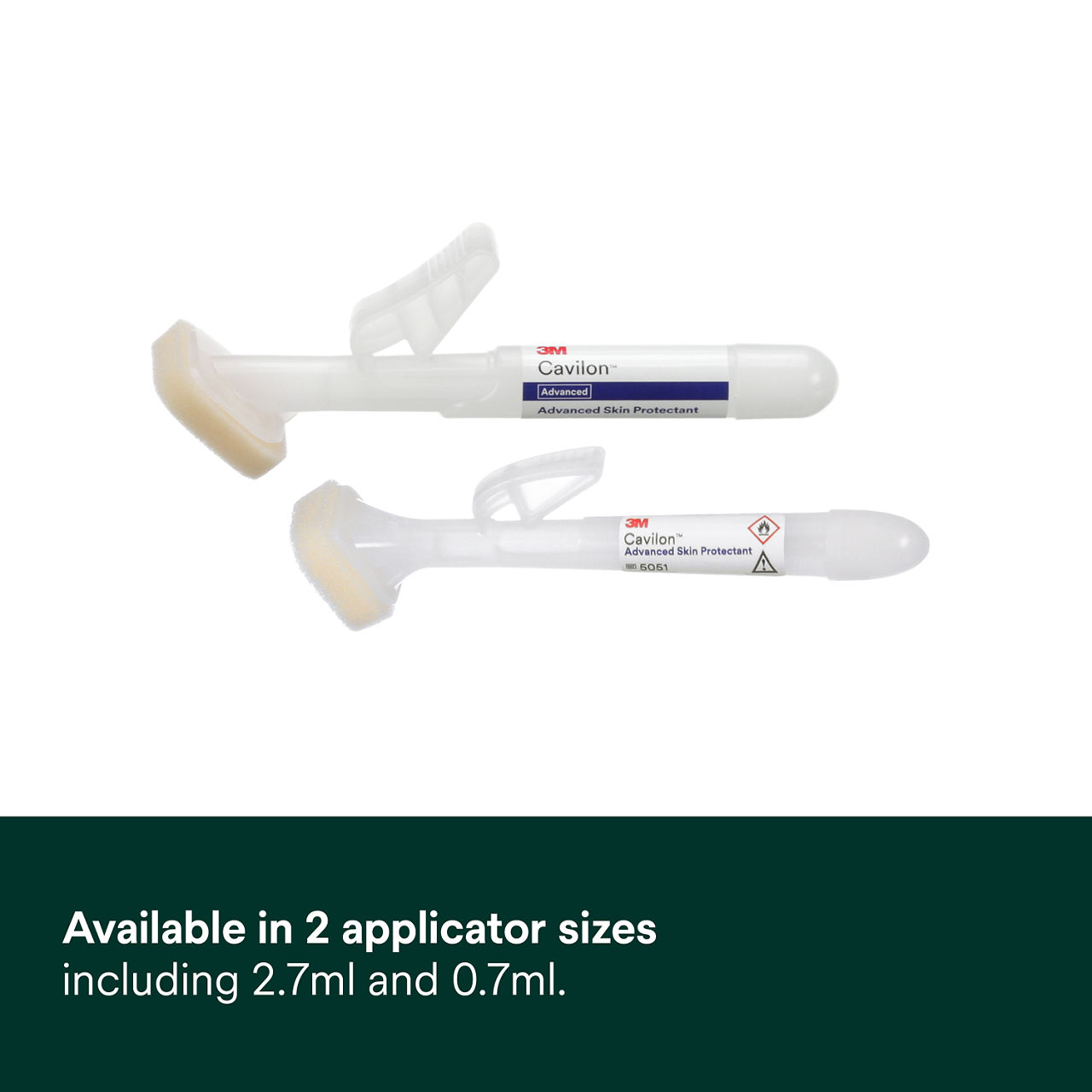
- Available in two sizes including 2.7 ml applicator coverage area of approx. up to 645 cm² and 0.7ml applicator coverage area of approx. 161.3 cm²
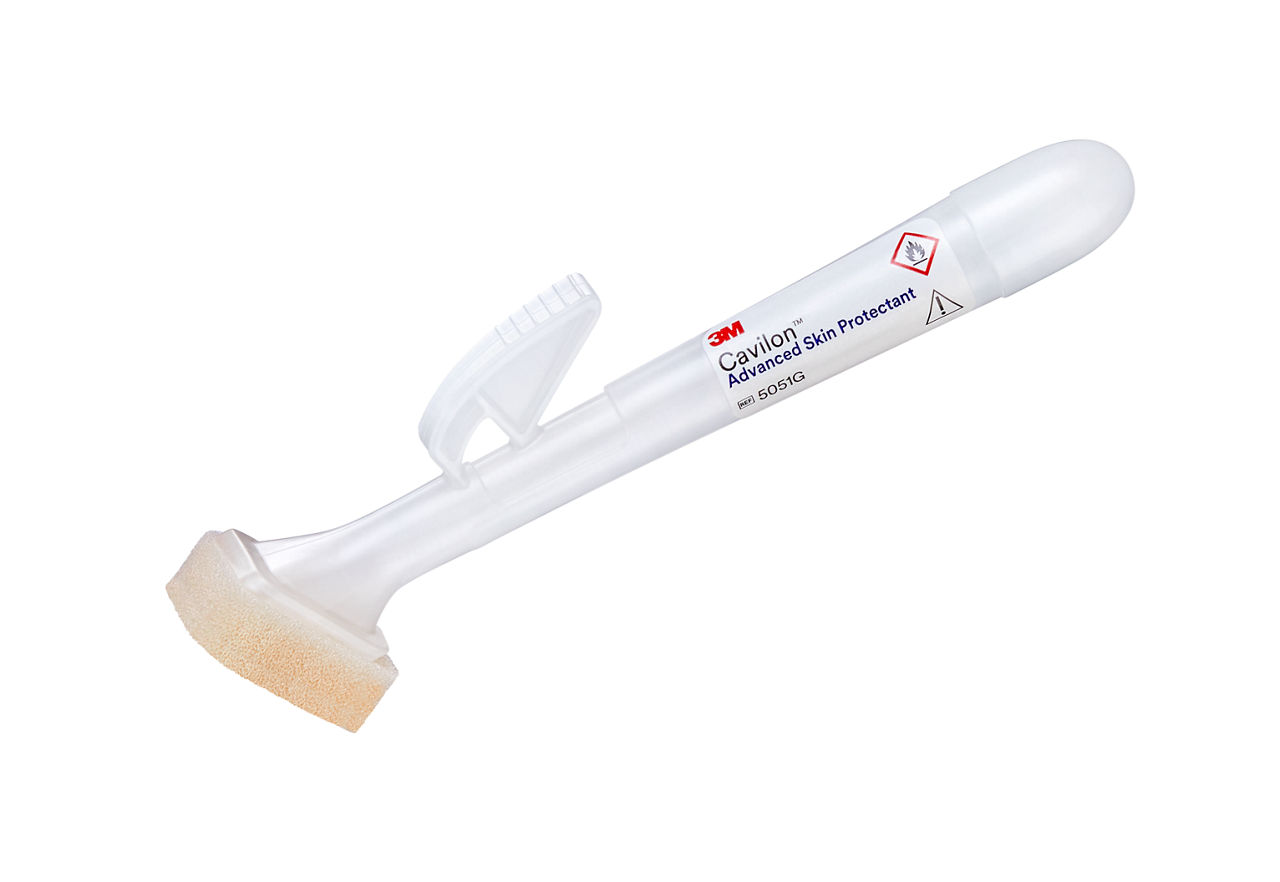
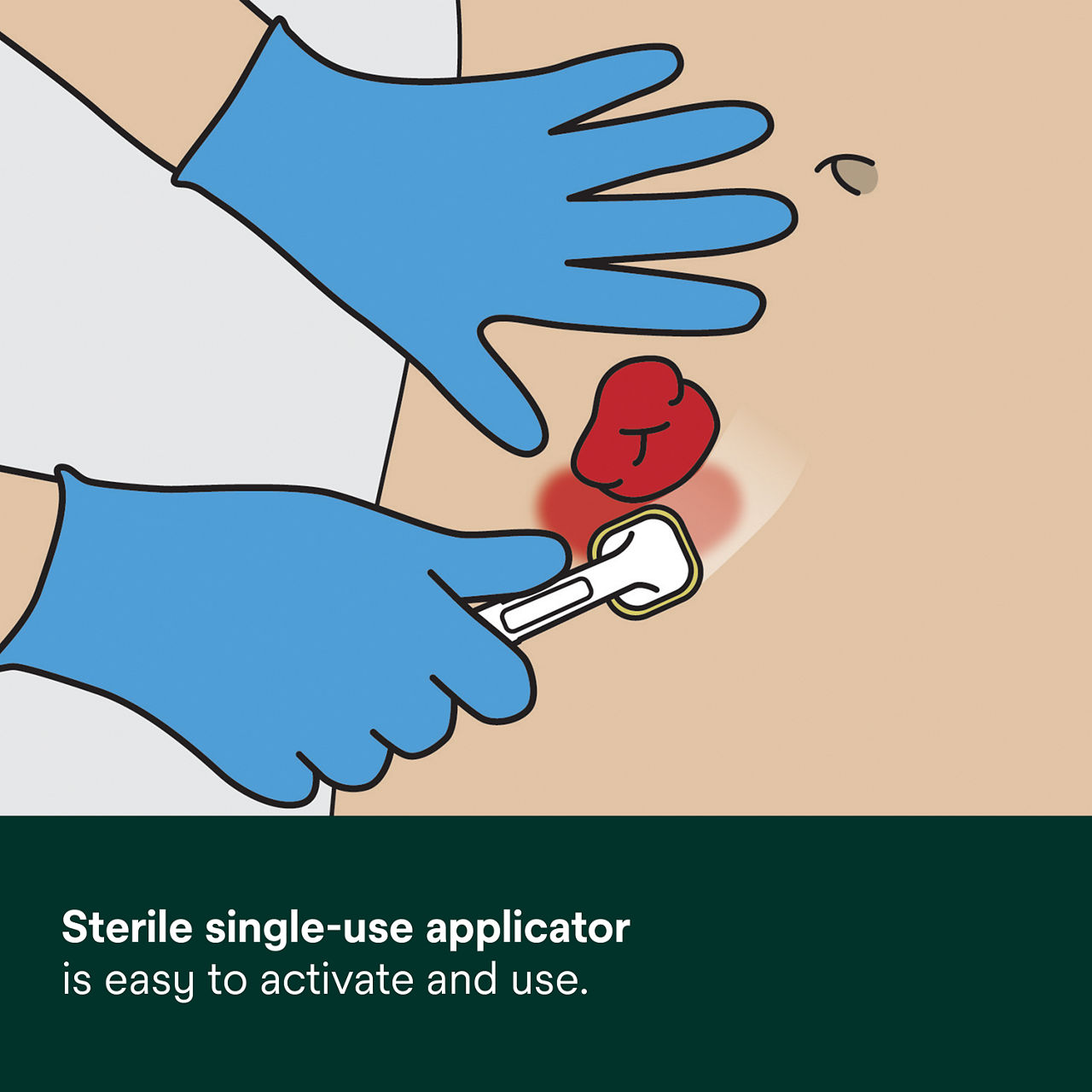
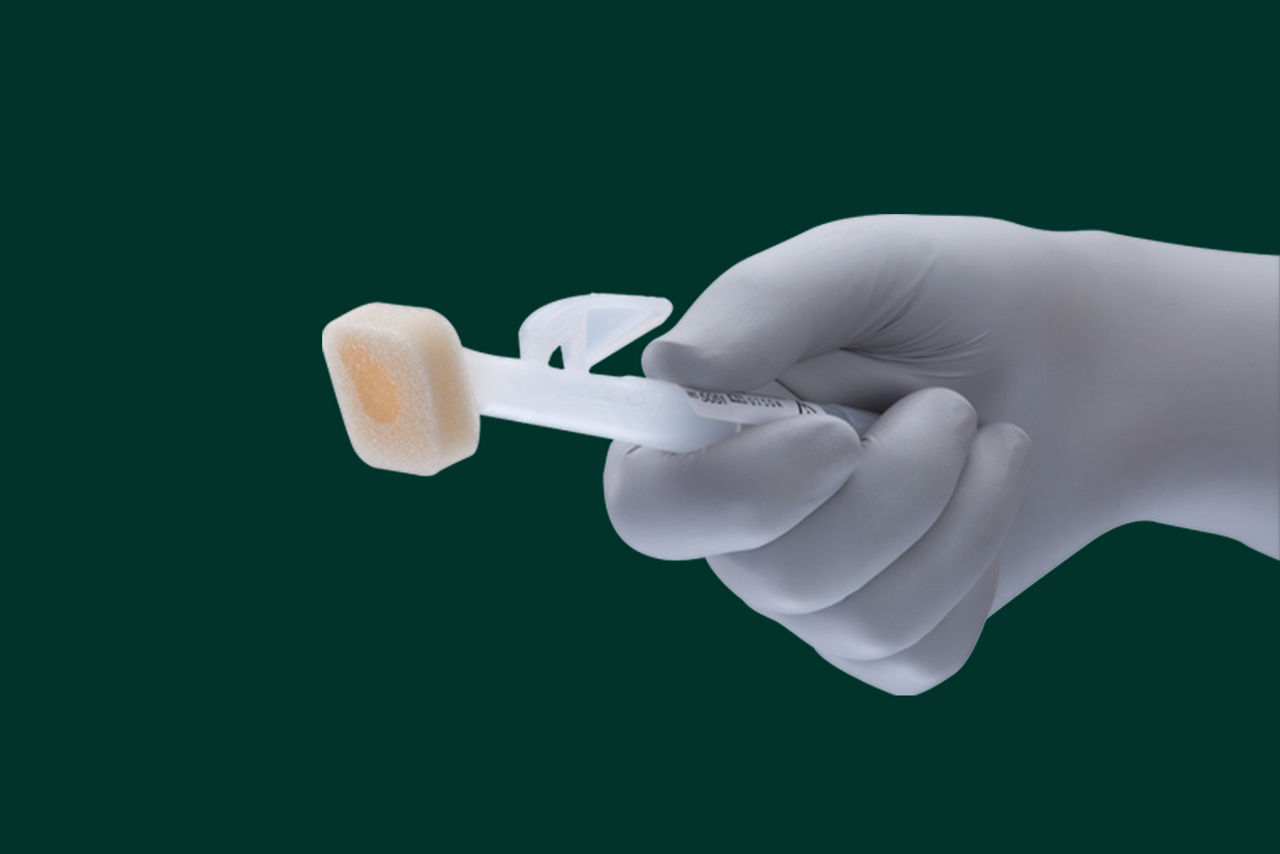
- Single-use applicator is easy to activate and use and allows for easy product dispensing
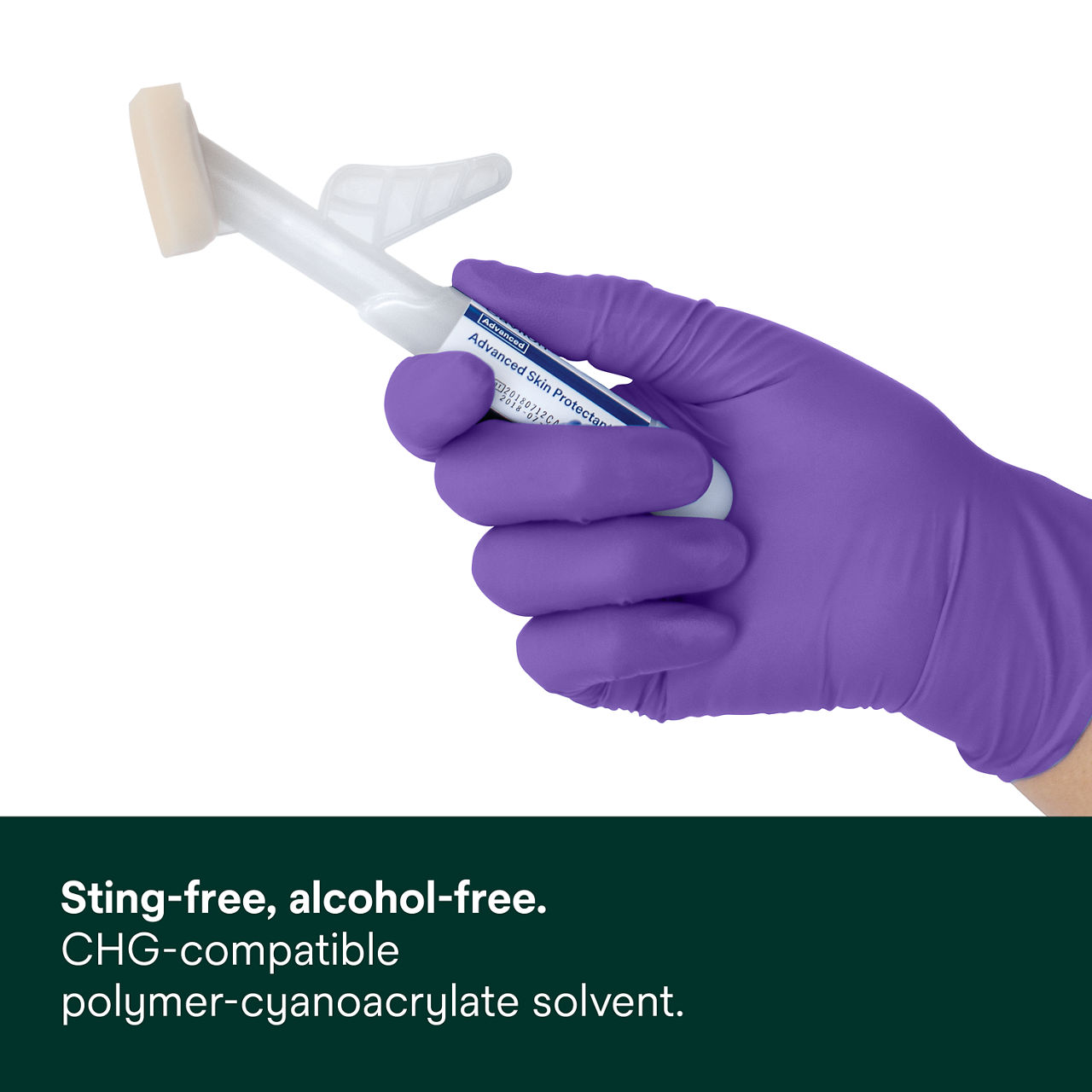
- Product dries in approximately 30 seconds and is chemically compatible with chlorhexidine gluconate (CHG), our acrylic negative pressure drapes and vascular access dressings*
*Refer to product IFU for full product guidance and use
- Forms a highly durable, long-lasting and unique elastomeric polymer barrier that helps prevent fracturing and cracking associated with pure cyanoacrylate films
- Does not diminish adherence of adhesive products (tapes, dressings, ostomy appliances)
- Use Cavilon Advanced skin protectant with Cavilon No-Rinse Skin Cleanser, Cavilon Bathing and Cleansing Wipes,our acrylic negative pressure drapes and vascular access dressins* to enhance your facility's skin protection program.*
*Refer to product IFU for full product guidance and use
Suggested Applications
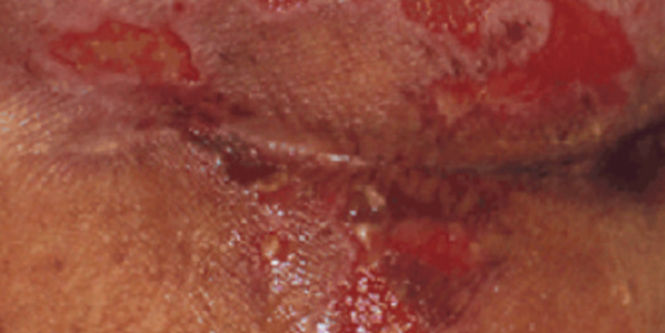
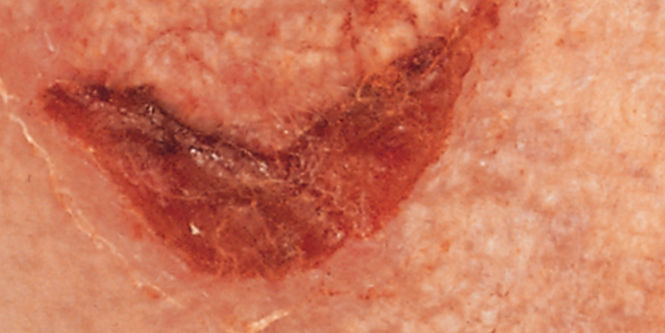
- Ideal for managing damaged or broken skin and protecting at-risk or compromised skin from MASD (including Incontinence-Associated Dermatitis, Peristomal Skin Damage, Periwound Skin Damage and Intertriginous Dermatitis (ITD) and irritants (such as liquid stool, mixed feces/urine, gastric fluid and chronic wound exudate moisture, friction and/or shear, a leading factor in pressure ulcer/injury development).
Legal
¹ Brennan, Mary R.; Milne, Catherine T.; Agrell-Kann, Marie; Ekholm, Bruce P. Clinical Evaluation of a Skin Protectant for the Management of Incontinence Associated Dermatitis: An Open-Label, Nonrandomized, Prospective Study. J of Wound, Ostomy & Continence Nursing. 2017. 44(2):172-180.Product specifications
| Brand |
Brand
Cavilon™ |
| Volume (Imperial) |
Volume (Imperial)
0.024 fl oz (us), 0.091 fl oz (us) |
| Volume (Metric) |
Volume (Metric)
0.7 ml, 2.7 ml |
| Category name |
Category name
Cyanoacrylate Skin Protectants |
| Delivery System |
Delivery System
Applicator |