Cavilon™ No Sting barriärfilm
Om produkten
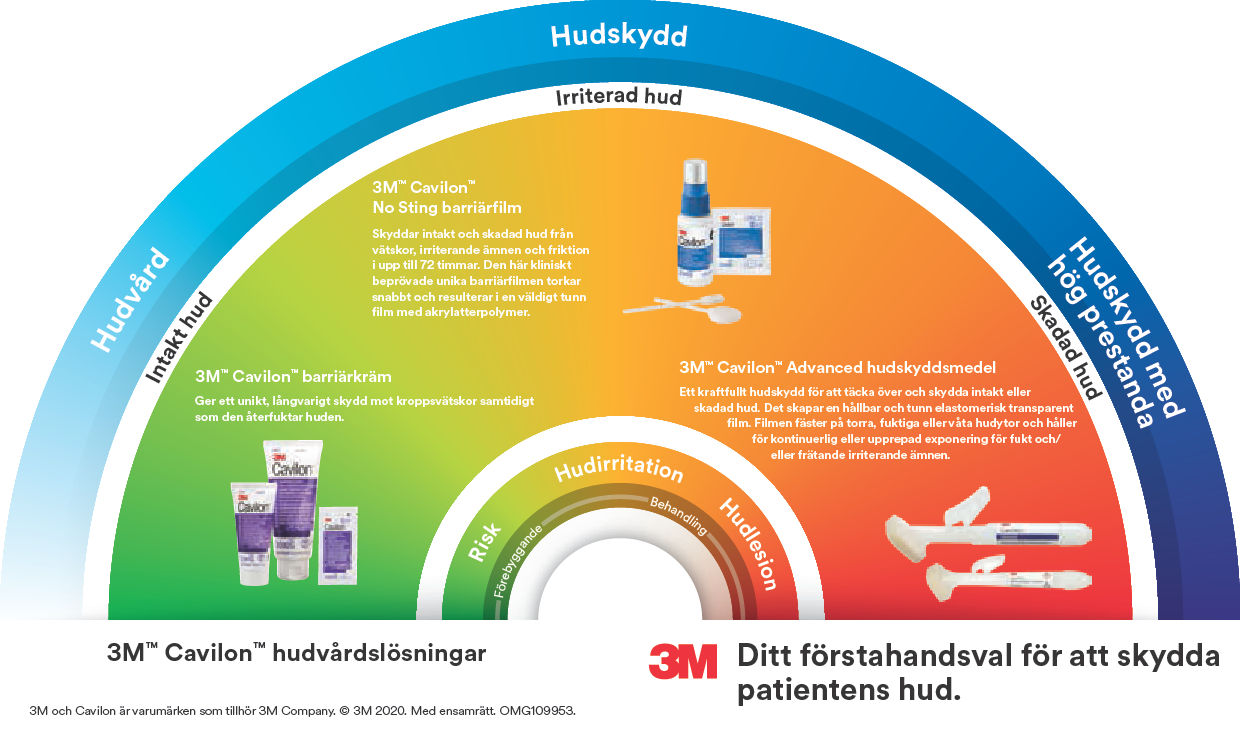
Cavilon No Sting barriärfilm är en alkoholfri flytande barriärfilm som torkar snabbt och bildar en andningsbar transparent film på huden.
Produktdetaljer
- Primär barriär mot kroppsvätskor, för förbättrad inkontinensvård som inte kräver borttagning
- Alkoholfri och svider inte, kan användas även på irriterad, skadad hud
- Säker, stör inte läkningsprocessen av sårig, skadad hud³ ⁴
- Snabbtorkande och klibbar inte, framtagen för att vara lätta att använda och ge optimal patientkomfort⁵
- Bevisad hjälp för att skydda huden under vård av accesspunkten⁶. Kompatibel med klorhexidinglukonat och povidonjod⁷
- Peel-open förpackning som underlättar aseptisk leveransteknik (endast 3343N)
- Medger att häftämnet fastnar
- En beprövad lösning med över 80 olika bevis som stöder dess effektivitet och kostnadsbesparingar⁸ ⁹
Föreslagna användningsområden
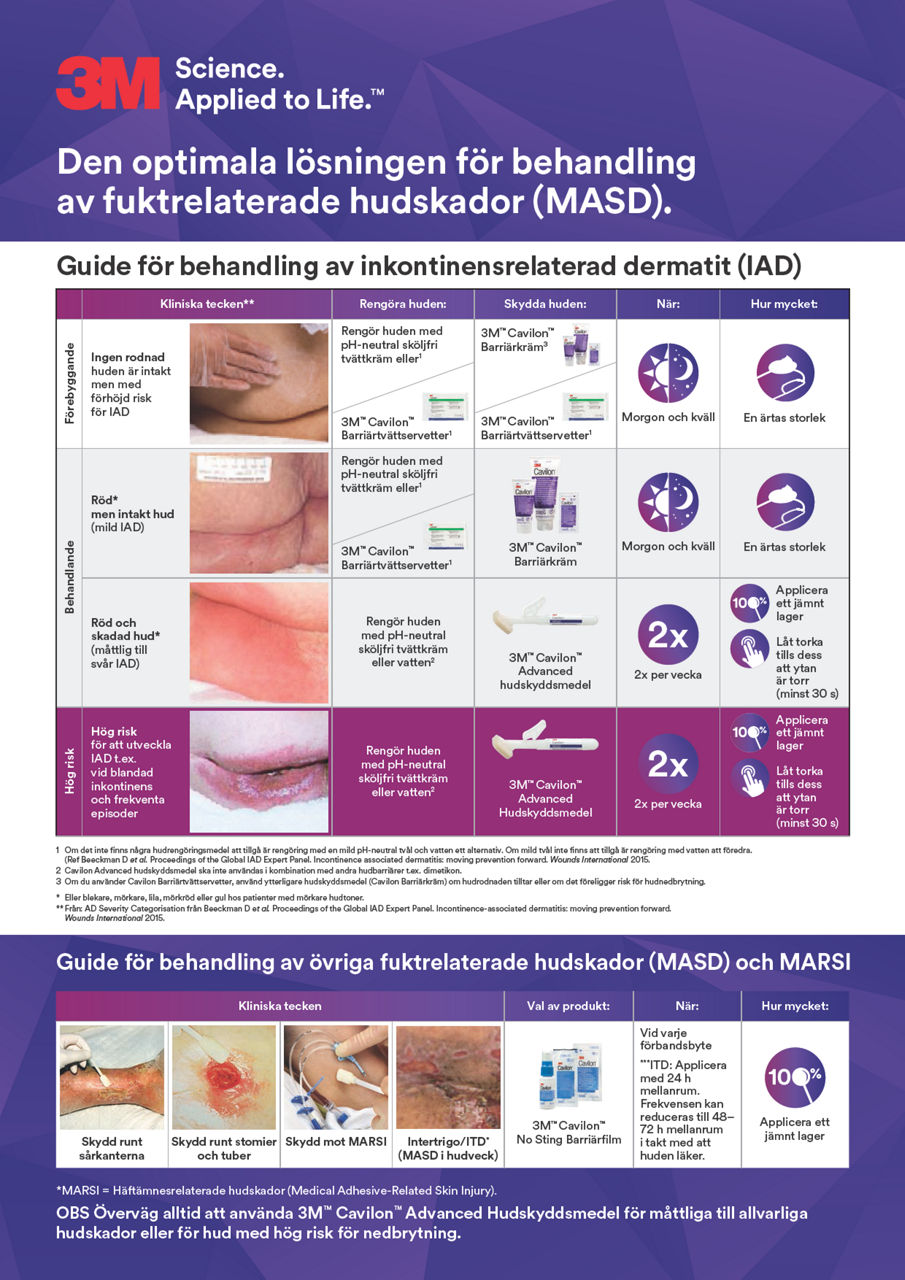
- Förebyggande av IAD (inkontinensrelaterad dermatit)⁸
- Skydd för peristomal/peritubulär hud⁸
- Skydd för huden runt såret⁸
- Förebyggande av häftämnesrelaterad hudskada (MARSI) vid intravenösa infarter, under förband, tejp och negativ trycksårsbehandling (NPWT)⁸
- Skydd mot fukt och friktion ¹⁰ (intertriginös dermatit)
Juridiskt
¹ 2010 Grove et al. Solventum White Paper. A Comparison of the Durability of Four Barrier Film Products Over a 72 Hour Period on Human Volunteers. ² CLIN-RPT-FINAL-ICH3-US-05-155867 EM-05-012107 Study to Determine the Ability of the Carbon Black Retention Method to Assess the Durability of Film-Forming Agents, Sponsor Final report (2010). ³ Cameron J, Hoffman D, Wilson J, Cherry G. “Comparison of two peri-wound skin protectants in venous leg ulcers: a randomized controlled trial”. J Wound Care, vol. 14, no. 5, 2005, pp. 233-236. ⁴ Rueda Lopez J, et.al. “A comparative study of a barrier product versus zinc oxide for the treatment of incontinent lesions”. Oral presentation at the World Union Wound Healing Society (WUWHS) Meeting in Paris; 2004. ⁵ CLIN-RPT-FINAL-INV-US-05-289804 EM-05-013869 Study to Assess the Durability of Film-Forming Barriers Using the Activated Carbon Retention Method (2016). ⁶ CLIN-SUPPORT-05-863013 NSBF Clinical Evidence Summary Brochure (2022). ⁷ Solventum data on file. ⁸ CLIN-SUPPORT-05-863013 NSBF Clinical Evidence Summary Brochure (2022). ⁹ CLIN-MISC-US-05-333303 Additional Clinical Evidence for NSBF (2017). ¹⁰ CLIN-MISC-US-05-169008 NSBF Friction Study Grove (2011)Produktspecifikationer
| Kategorinamn |
Kategorinamn
No-Sting barriärfilmer |
| VolumeMetric |
VolumeMetric
28 ml |
| Märke |
Märke
Cavilon™ |
| Volym |
Volym
28 ml, 1 ml, 3 ml |
| Volym (Imperial) |
Volym (Imperial)
0.947 fl oz (us), 0.034 fl oz (us), 0.101 fl oz (us) |
| Trademark2 |
Trademark2
Cavilon™ |
| Förpackningar |
Förpackningar
Spray, Munstycke |