Tegaderm™ CHG Chlorhexidine Gluconate I.V. Port Dressing 1665R
About the product
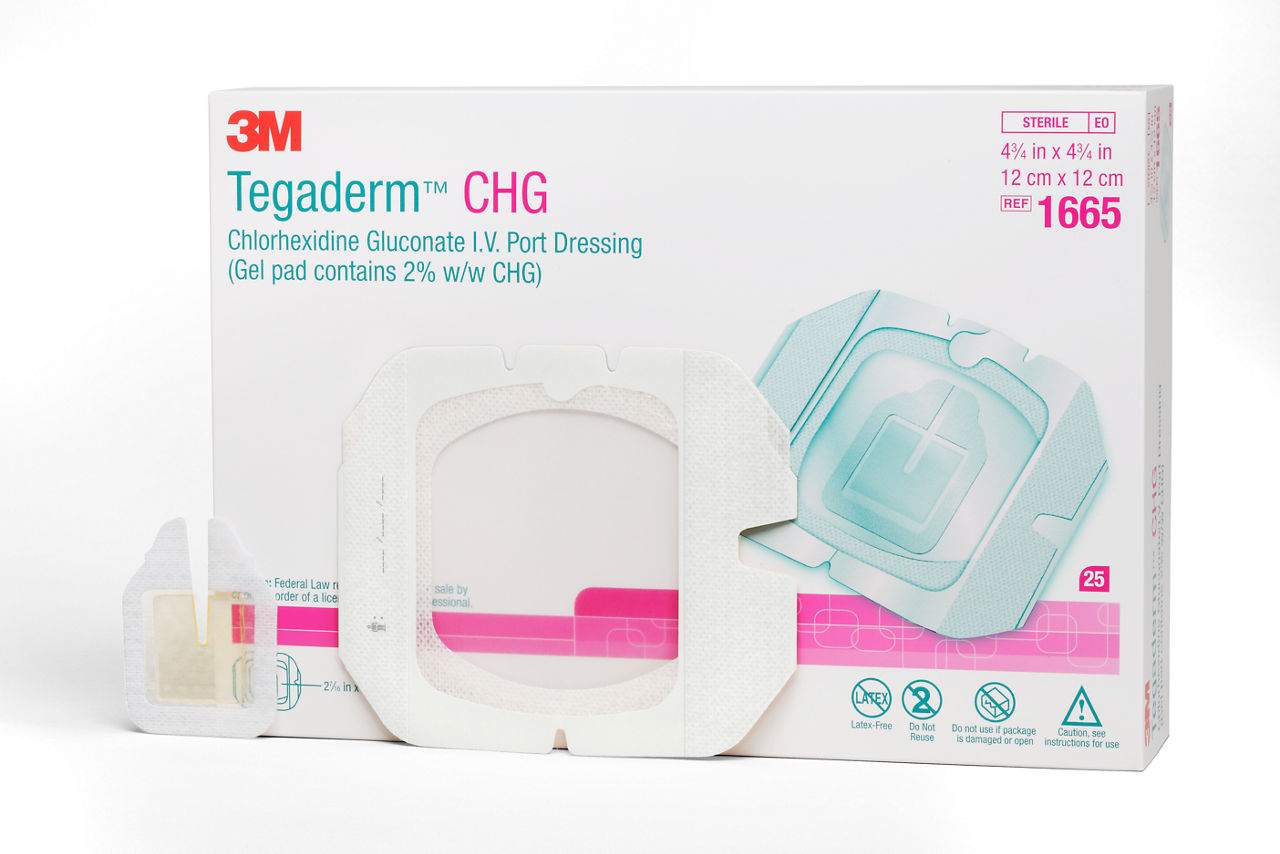
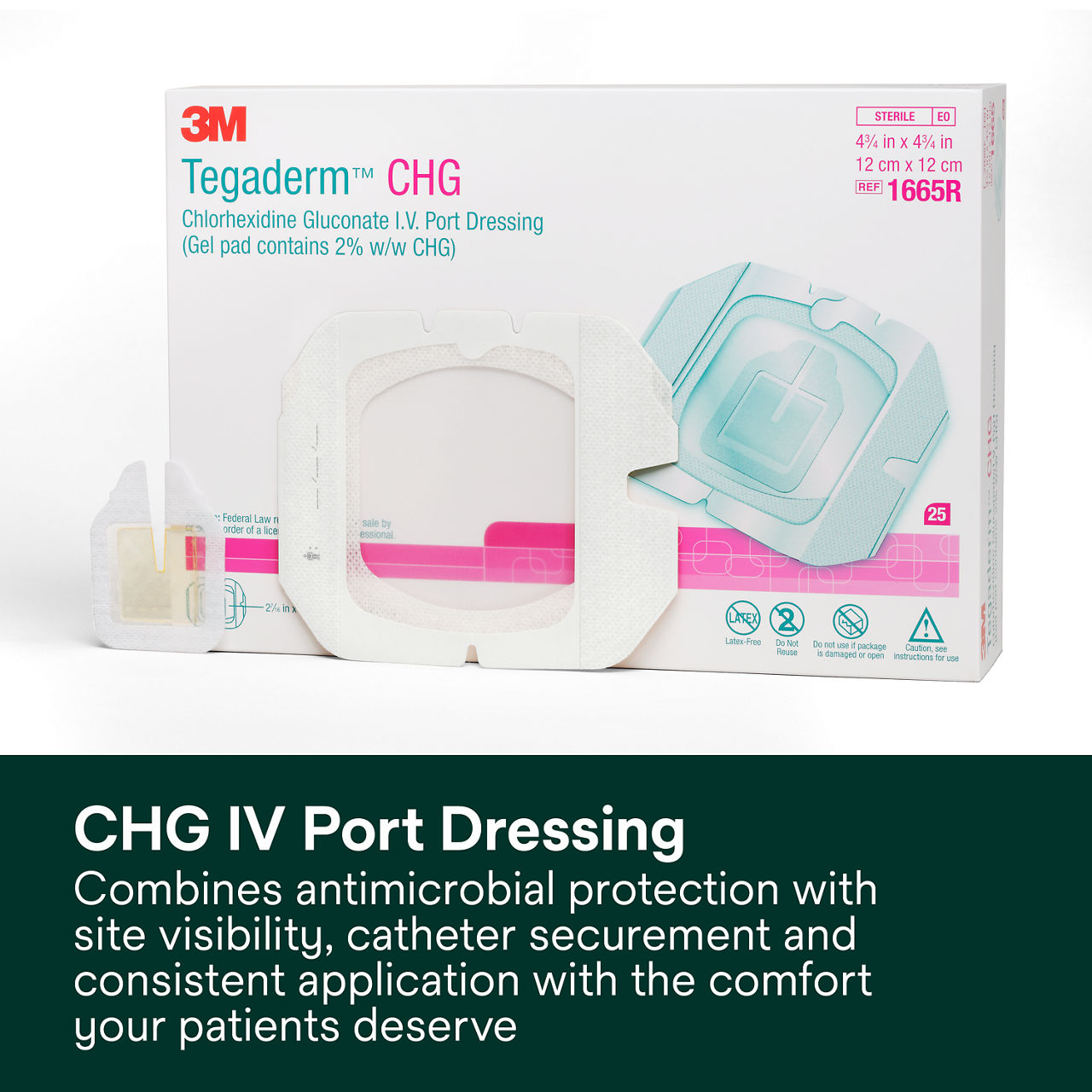
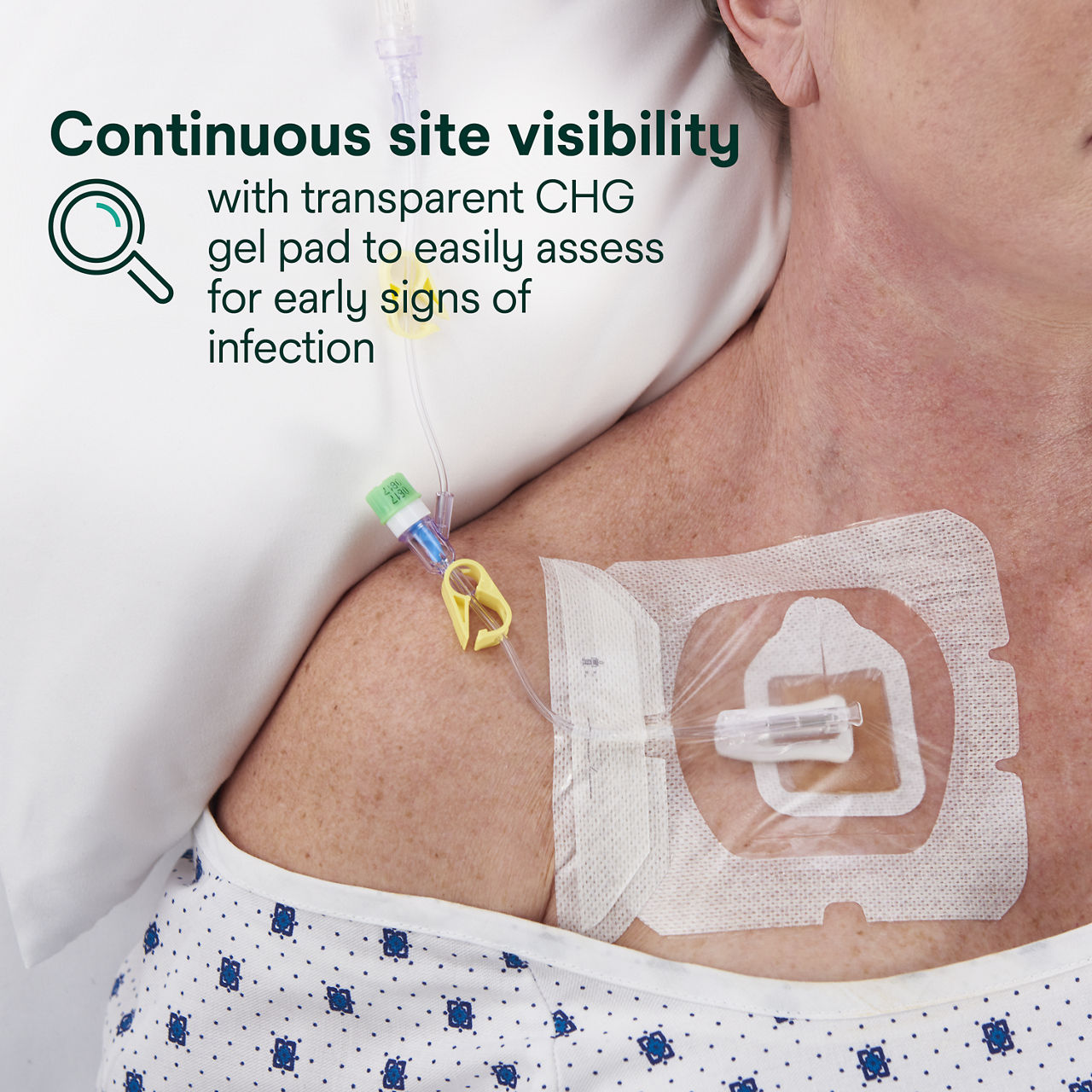
The Tegaderm CHG Chlorhexidine Gluconate I.V. port dressing, 1665R, is an antimicrobial (CHG) gel pad plus IV port dressing specifically designed to protect single or double implanted venous portsand non-coring needles. It combines antimicrobial protection with site visibility, catheter securement and consistent applicationfor patients with implanted venous ports, including oncology and haematology patients.
Product details
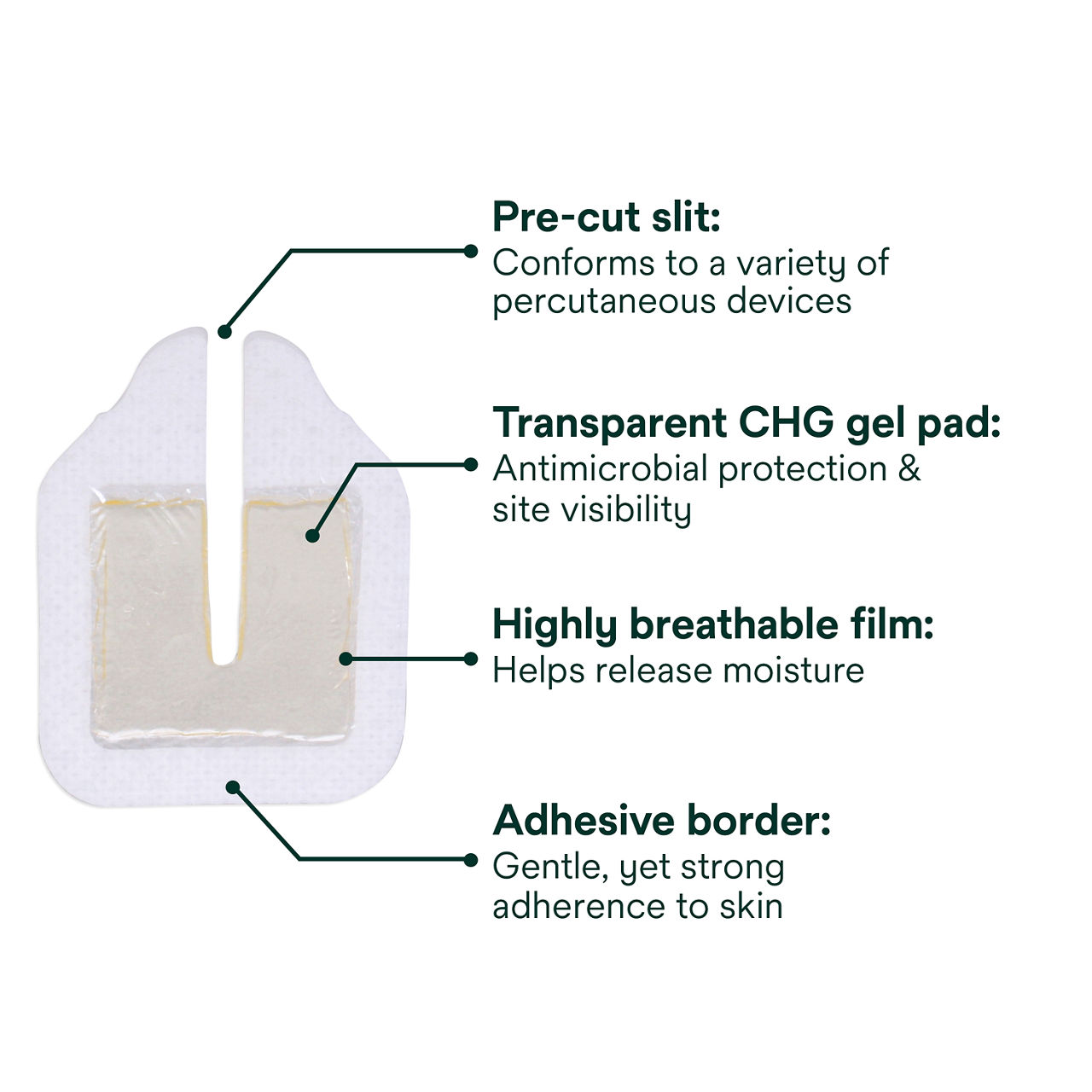
Tegaderm CHG Chlorhexidine Gluconate I.V. Port Dressing contains an antimicrobial (CHG) gel pad plus an IV port dressing. TegadermTM CHG is intended to reduce catheter-related bloodstream infections (CRBSI) in patients with central venous or arterial catheters. The transparent CHG gel pad provides site visibility and immediate and continuous antimicrobial protection against microorganisms associated with CRBSIs for up to 7 days without additional moisture required to activate. The adhesive border provides gentle, yet strong adherence to skin, and the pre-cut slit in the gel pad is designed to conform around a variety of percutaneous devices. The gel pad remains clear and protects even in the presence of blood, saline and exudates.
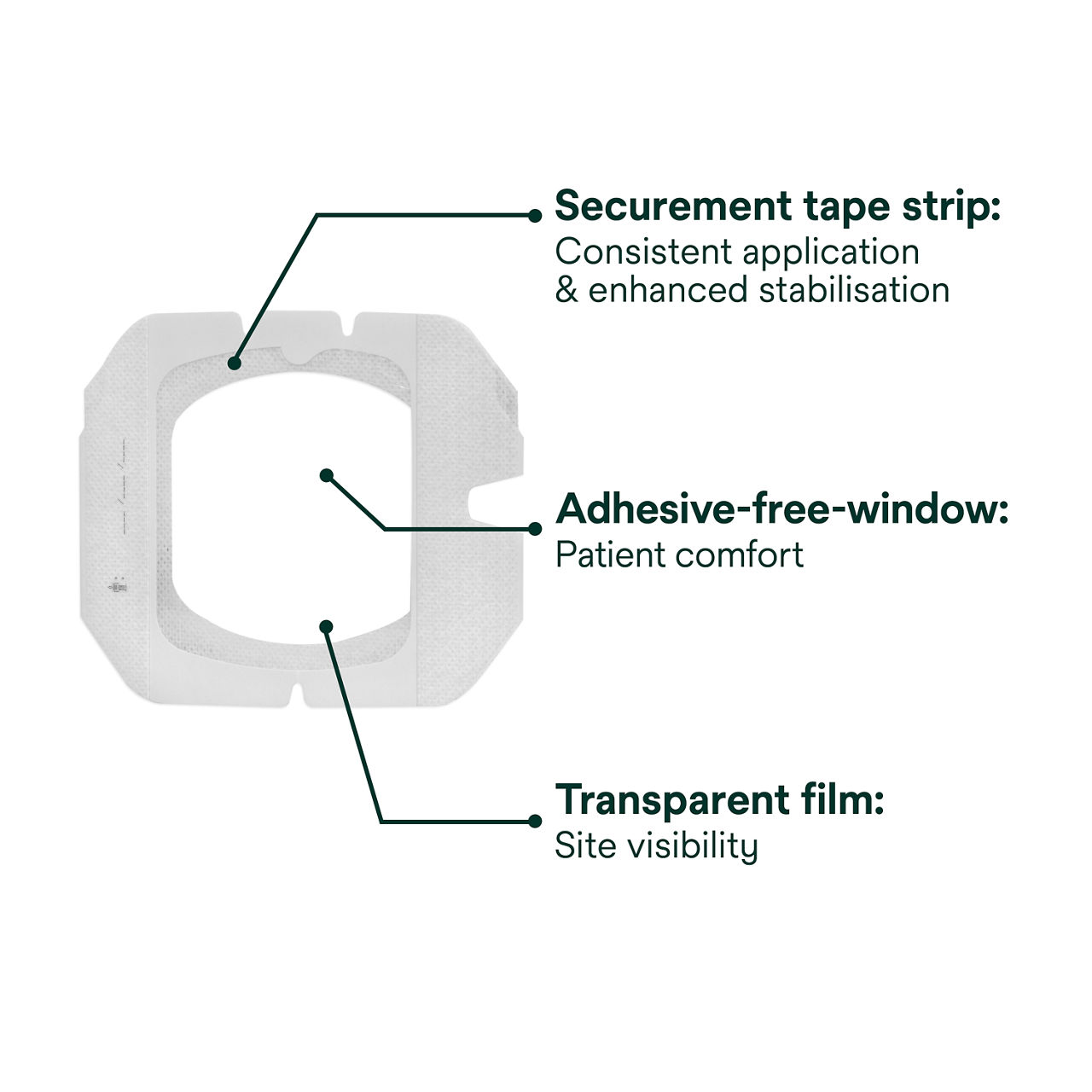
The IV port dressing has an adhesive-free window to reduce the risk of the dressing sticking to the needle during removal. The conforming edge border with pattern-coated adhesive is designed to reduce edge lift and support longer wear time. A securement tape strip with a notch seals the dressing border and anchors tubing, and a documentation tape strip preprinted for documenting dressing changes provides additional securement.
The CHG gel pad and cover dressing are also sold separately.
*In vitro testing shows that the film provides a barrier against viruses 27 nm in diameter or larger while the dressing remains intact without leakage.
- IV port dressing plus CHG gel pad offers antimicrobial protection with the comfort your patients deserve.
- Tegaderm CHG I.V. Dressing is intended to reduce catheter-related bloodstream infections (CRBSIs) in patients with central venous or arterial catheters.
- CHG gel pad provides immediate and continuous antimicrobial activity – reducing skin flora and preventing its regrowth – for up to 7 days.
- Dressing is easy to remove with adhesive-free window minimising adhesive contact with non-coring needle and patient’s skin.
- Transparent dressing and CHG gel pad allow continuous site visibility to easily assess for early signs of infection.
- CHG gel pad conforms around the needle and stays in contact with the skin.
- Accommodates implanted venous ports and a variety of non-coring Huber needles.
- Picture-frame delivery makes placement accurate and easy, minimising potential to stick to gloves or to itself.
Suggested Applications
- To cover, protect and secure needles inserted in implanted ports on oncology and haematology patients
- To apply antimicrobial protection to implanted ports on oncology and haematology patients
Legal
* In vitro testing shows that the film provides a barrier against viruses 27 nm in diameter or larger while the dressing remains intact without leakage.Product specifications
| Global Catalog Number |
Global Catalog Number
1665R |
| Brand |
Brand
Tegaderm™ |
| Overall Length (Imperial) |
Overall Length (Imperial)
4.72 in |
| Overall Width (Metric) |
Overall Width (Metric)
12 cm |
| Overall Length (Metric) |
Overall Length (Metric)
12 cm |
| Category name |
Category name
Antimicrobial IV Dressings |
| Overall Width (Imperial) |
Overall Width (Imperial)
4.72 in |