3M™ Cavilon™ No Sting Barrier Film, 3343E, 1 mL Wand, Sterile, 25/CAR, 4CAR/CS, 100/CS
About the product
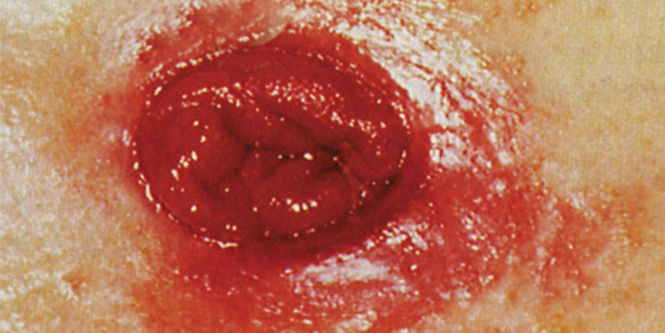
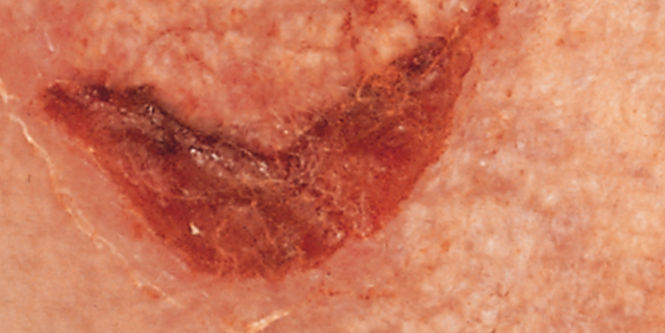
Help prevent skin damage before it occurs with 3M™ Cavilon™ No Sting Barrier Film. It’s the original and only terpolymer-based alcohol-free* barrier film that creates a gentle, breathable, waterproof and transparent coating on the skin for 72 hours of protection against friction, shear, adhesives and body fluids.
*Of leading competitors in the market, based on disclosed ingredient information.
Product details
Offering a variety of options including a wand, wipe and spray, Cavilon No Sting Barrier Film is convenient and easy to apply. It comes in an easy-to-open peel-down package that allows for aseptic delivery* and contains a unique blend of polymer and silicone fluid (or silicone oligomer). The additional silicone allows the film to flex with the skin and helps to maintain a continuous protective coating.
*Only applicable with 3343 products.
- Unique – The original and only terpolymer-based alcohol-free barrier film* that helps prevent skin damage before it occurs
*Of leading competitors in the market, based on disclosed ingredient information.
- Proven – Supported by more evidence than any other moisture barrier or barrier film, 80+ studies
- Versatile – Solution for many skin problems including medical adhesive-related skin injury (MARSI), periwound skin damage, peristomal skin damage, incontinence-associated dermatitis, and other moisture and friction skin damage prevention needs
- Gentle – Alcohol-free, low dermatitis potential formula won’t sting or irritate skin and can be used on intact or damaged skin
- Durable – Fast-drying, long-lasting, waterproof barrier film doesn’t wash off, making it easy for clinicians to use
- Effective – Continuous sterile* skin protection compatible with chlorhexidine gluconate (CHG), making it essential for vascular access site protection.
*Wands and wipes only.
Suggested Applications
- Protection from moisture and friction (MASD).
- Peristomal/peritube skin protection.
- MARSI prevention around I.V. sites and under dressings, tapes and negative pressure wound therapy (NPWT).
- Helps prevent incontinence-associated dermatitis (IAD).
Legal
¹ 2010 Grove et al. Solventum White Paper. A Comparison of the Durability of Four Barrier Film Products Over a 72 Hour Period on Human Volunteers. ² CLIN-RPT-FINAL-ICH3-US-05-155867 EM-05-012107 Study to Determine the Ability of the Carbon Black Retention Method to Assess the Durability of Film-Forming Agents, Sponsor Final report (2010). ³ Cameron J, Hoffman D, Wilson J, Cherry G. “Comparison of two peri-wound skin protectants in venous leg ulcers: a randomized controlled trial”. J Wound Care, vol. 14, no. 5, 2005, pp. 233-236. ⁴ Rueda Lopez J, et.al. “A comparative study of a barrier product versus zinc oxide for the treatment of incontinent lesions”. Oral presentation at the World Union Wound Healing Society (WUWHS) Meeting in Paris; 2004. ⁵ CLIN-RPT-FINAL-INV-US-05-289804 EM-05-013869 Study to Assess the Durability of Film-Forming Barriers Using the Activated Carbon Retention Method (2016). ⁶ CLIN-SUPPORT-05-863013 NSBF Clinical Evidence Summary Brochure (2022). ⁷ Solventum data on file. ⁸ CLIN-SUPPORT-05-863013 NSBF Clinical Evidence Summary Brochure (2022). ⁹ CLIN-MISC-US-05-333303 Additional Clinical Evidence for NSBF (2017). ¹⁰ CLIN-MISC-US-05-169008 NSBF Friction Study Grove (2011)Product specifications
| Brand |
Brand
Cavilon™ |
| Volume (Imperial) |
Volume (Imperial)
0.034 fl oz (us) |
| Volume (Metric) |
Volume (Metric)
1 ml |
| Category name |
Category name
No-Sting Barrier Films |
| Delivery System |
Delivery System
Wand |